Input interpretation

non-polar solvents | relative molecular mass
Summary
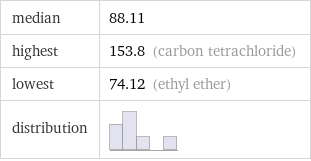
median | 88.11 highest | 153.8 (carbon tetrachloride) lowest | 74.12 (ethyl ether) distribution |
Ranked values
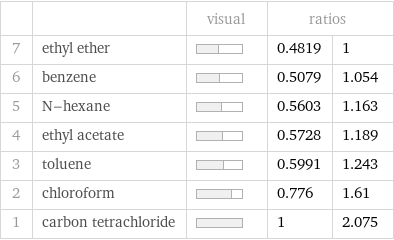
| | visual | ratios | 7 | ethyl ether | | 0.4819 | 1 6 | benzene | | 0.5079 | 1.054 5 | N-hexane | | 0.5603 | 1.163 4 | ethyl acetate | | 0.5728 | 1.189 3 | toluene | | 0.5991 | 1.243 2 | chloroform | | 0.776 | 1.61 1 | carbon tetrachloride | | 1 | 2.075
Relative molecular mass rankings
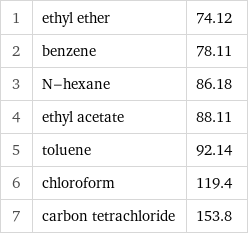
1 | ethyl ether | 74.12 2 | benzene | 78.11 3 | N-hexane | 86.18 4 | ethyl acetate | 88.11 5 | toluene | 92.14 6 | chloroform | 119.4 7 | carbon tetrachloride | 153.8
Comparisons for median relative molecular mass 88.11

≈ ( 0.12 ≈ 1/8 ) × relative molecular mass of fullerene ( ≈ 721 )

≈ 0.45 × relative molecular mass of caffeine ( ≈ 194 )
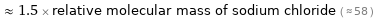
≈ 1.5 × relative molecular mass of sodium chloride ( ≈ 58 )
Corresponding quantities
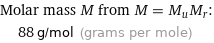
Molar mass M from M = M_uM_r: | 88 g/mol (grams per mole)

Molecular mass m from m = M_rM_u/N_A: | 1.5×10^-22 grams | 1.5×10^-25 kg (kilograms)