Input interpretation

metallic elements | specific heat at STP
Summary
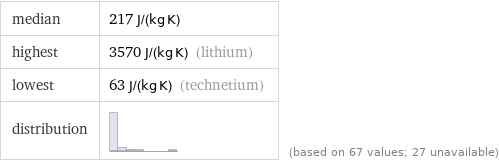
median | 217 J/(kg K) highest | 3570 J/(kg K) (lithium) lowest | 63 J/(kg K) (technetium) distribution | | (based on 67 values; 27 unavailable)
Entities with missing values

promethium | polonium | francium | neptunium | plutonium | americium | curium | berkelium | californium | einsteinium | ... (total: 27)
Distribution plots
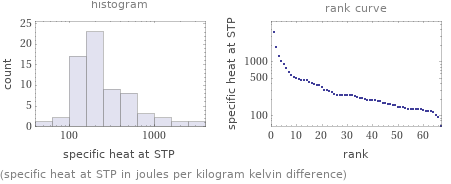
(specific heat at STP in joules per kilogram kelvin difference)
Specific heat at STP rankings

1 | technetium | 63 J/(kg K) 2 | radium | 92 J/(kg K) 3 | protactinium | 99.1 J/(kg K) 4 | uranium | 116 J/(kg K) 5 | thorium | 118 J/(kg K) 6 | actinium | 120 J/(kg K) 7 | bismuth | 122 J/(kg K) 8 | lead | 127 J/(kg K) 9 | thallium | 129 J/(kg K) 10 | gold | 129.1 J/(kg K) ⋮ | | 58 | vanadium | 489 J/(kg K) 59 | titanium | 520 J/(kg K) 60 | scandium | 567 J/(kg K) 61 | calcium | 631 J/(kg K) 62 | potassium | 757 J/(kg K) 63 | aluminum | 904 J/(kg K) 64 | magnesium | 1020 J/(kg K) 65 | sodium | 1230 J/(kg K) 66 | beryllium | 1820 J/(kg K) 67 | lithium | 3570 J/(kg K) (based on 67 values; 27 unavailable)
Unit conversions for median specific heat at STP 217 J/(kg K)
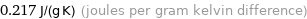
0.217 J/(g K) (joules per gram kelvin difference)
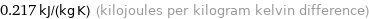
0.217 kJ/(kg K) (kilojoules per kilogram kelvin difference)
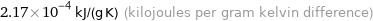
2.17×10^-4 kJ/(g K) (kilojoules per gram kelvin difference)

217 J/(kg °C) (joules per kilogram degree Celsius difference)
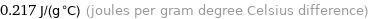
0.217 J/(g °C) (joules per gram degree Celsius difference)

0.0518 BTU_IT/(lb °F) (IT British thermal units per pound degree Fahrenheit difference)
Comparisons for median specific heat at STP 217 J/(kg K)
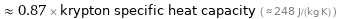
≈ 0.87 × krypton specific heat capacity ( ≈ 248 J/(kg K) )
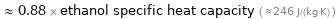
≈ 0.88 × ethanol specific heat capacity ( ≈ 246 J/(kg K) )
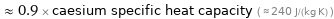
≈ 0.9 × caesium specific heat capacity ( ≈ 240 J/(kg K) )
Thermodynamic properties
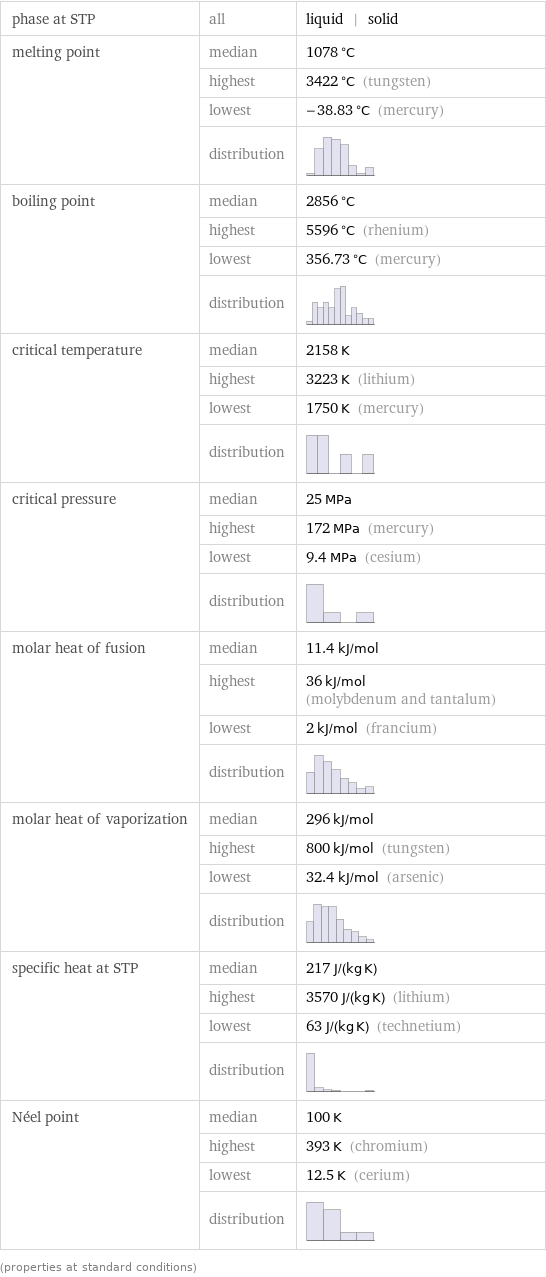
phase at STP | all | liquid | solid melting point | median | 1078 °C | highest | 3422 °C (tungsten) | lowest | -38.83 °C (mercury) | distribution | boiling point | median | 2856 °C | highest | 5596 °C (rhenium) | lowest | 356.73 °C (mercury) | distribution | critical temperature | median | 2158 K | highest | 3223 K (lithium) | lowest | 1750 K (mercury) | distribution | critical pressure | median | 25 MPa | highest | 172 MPa (mercury) | lowest | 9.4 MPa (cesium) | distribution | molar heat of fusion | median | 11.4 kJ/mol | highest | 36 kJ/mol (molybdenum and tantalum) | lowest | 2 kJ/mol (francium) | distribution | molar heat of vaporization | median | 296 kJ/mol | highest | 800 kJ/mol (tungsten) | lowest | 32.4 kJ/mol (arsenic) | distribution | specific heat at STP | median | 217 J/(kg K) | highest | 3570 J/(kg K) (lithium) | lowest | 63 J/(kg K) (technetium) | distribution | Néel point | median | 100 K | highest | 393 K (chromium) | lowest | 12.5 K (cerium) | distribution | (properties at standard conditions)