Input interpretation
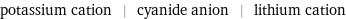
potassium cation | cyanide anion | lithium cation
Structure diagrams
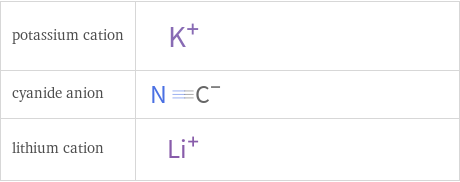
Structure diagrams
General properties
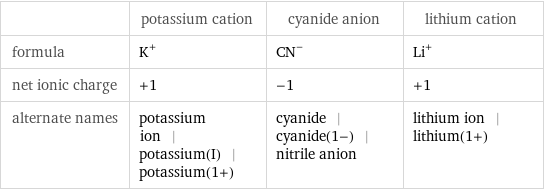
| potassium cation | cyanide anion | lithium cation formula | K^+ | (CN)^- | Li^+ net ionic charge | +1 | -1 | +1 alternate names | potassium ion | potassium(I) | potassium(1+) | cyanide | cyanide(1-) | nitrile anion | lithium ion | lithium(1+)
Ionic radii
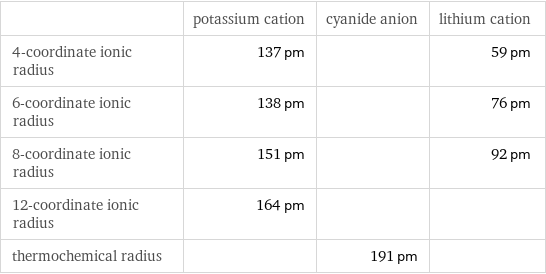
| potassium cation | cyanide anion | lithium cation 4-coordinate ionic radius | 137 pm | | 59 pm 6-coordinate ionic radius | 138 pm | | 76 pm 8-coordinate ionic radius | 151 pm | | 92 pm 12-coordinate ionic radius | 164 pm | | thermochemical radius | | 191 pm |
Units

Other properties
| potassium cation | cyanide anion | lithium cation ion class | alkali metal ions | biomolecule ions | cations | group 1 ions | monatomic cations | s block ions | anions | polyatomic ions | alkali metal ions | biomolecule ions | cations | group 1 ions | monatomic cations | s block ions common sources of ion | potassium vinyltrifluoroborate (1 eq) | potassium tungstate (2 eq) | potassium trimethylsilanolate (1 eq) | potassium trifluoroacetate (1 eq) | potassium thioacetate (1 eq) | zinc cyanide (2 eq) | tetraethylammonium cyanide (1 eq) | tetrabutylammonium cyanide (1 eq) | sodium dicyanoaurate (2 eq) | sodium cyanide (1 eq) | silver cyanide (1 eq) | potassium tetracyanoplatinate(II) (4 eq) | potassium tetracyanonickelate(II) (4 eq) | potassium hexacyanoplatinate(IV) (6 eq) | zonyl®FSA fluorosurfactant (1 eq) | R-alpine-hydride™ (1 eq)