Input interpretation
lead(II) cyanamide | rhenium
Chemical names and formulas
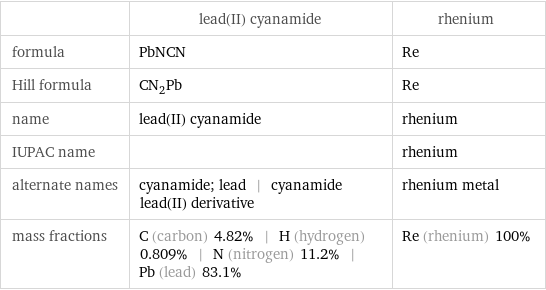
| lead(II) cyanamide | rhenium formula | PbNCN | Re Hill formula | CN_2Pb | Re name | lead(II) cyanamide | rhenium IUPAC name | | rhenium alternate names | cyanamide; lead | cyanamide lead(II) derivative | rhenium metal mass fractions | C (carbon) 4.82% | H (hydrogen) 0.809% | N (nitrogen) 11.2% | Pb (lead) 83.1% | Re (rhenium) 100%
Structure diagrams
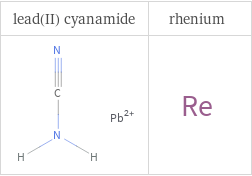
Structure diagrams
Basic properties
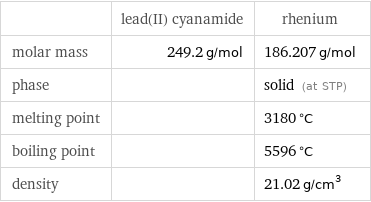
| lead(II) cyanamide | rhenium molar mass | 249.2 g/mol | 186.207 g/mol phase | | solid (at STP) melting point | | 3180 °C boiling point | | 5596 °C density | | 21.02 g/cm^3
Units

Thermodynamic properties
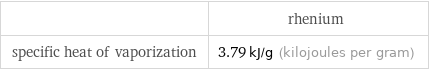
| rhenium specific heat of vaporization | 3.79 kJ/g (kilojoules per gram)
Solid properties
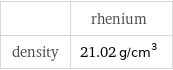
| rhenium density | 21.02 g/cm^3
Units
