Input interpretation

solid compounds | molar heat of vaporization
Summary

median | 295 kJ/mol highest | 453 kJ/mol (vanadium) lowest | 41.1 kJ/mol (acetoxime) distribution | | (based on 14 values; 6 unavailable)
Entities with missing values
beryllium oxide | lime | potassium fluoride | quartz | sodium amide | sodium fluoride (total: 6)
Distribution plots
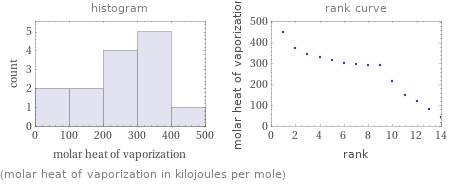
(molar heat of vaporization in kilojoules per mole)
Molar heat of vaporization rankings
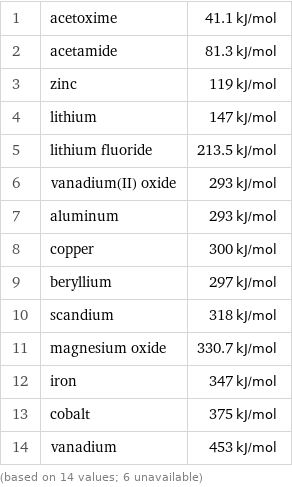
1 | acetoxime | 41.1 kJ/mol 2 | acetamide | 81.3 kJ/mol 3 | zinc | 119 kJ/mol 4 | lithium | 147 kJ/mol 5 | lithium fluoride | 213.5 kJ/mol 6 | vanadium(II) oxide | 293 kJ/mol 7 | aluminum | 293 kJ/mol 8 | copper | 300 kJ/mol 9 | beryllium | 297 kJ/mol 10 | scandium | 318 kJ/mol 11 | magnesium oxide | 330.7 kJ/mol 12 | iron | 347 kJ/mol 13 | cobalt | 375 kJ/mol 14 | vanadium | 453 kJ/mol (based on 14 values; 6 unavailable)
Unit conversions for median molar heat of vaporization 295 kJ/mol
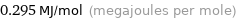
0.295 MJ/mol (megajoules per mole)
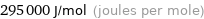
295000 J/mol (joules per mole)

70.5 kcal/mol (thermochemical kilocalories per mole)
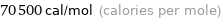
70500 cal/mol (calories per mole)
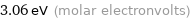
3.06 eV (molar electronvolts)