Input interpretation
rubidium sulfate
Chemical names and formulas

formula | Rb_2SO_4 Hill formula | O_4Rb_2S_1 name | rubidium sulfate alternate names | rubidium; sulfuric acid mass fractions | O (oxygen) 24% | Rb (rubidium) 64% | S (sulfur) 12%
Structure diagram
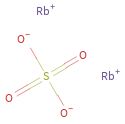
Structure diagram
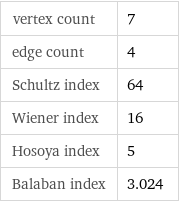
vertex count | 7 edge count | 4 Schultz index | 64 Wiener index | 16 Hosoya index | 5 Balaban index | 3.024
Basic properties
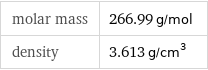
molar mass | 266.99 g/mol density | 3.613 g/cm^3
Units

Thermodynamic properties
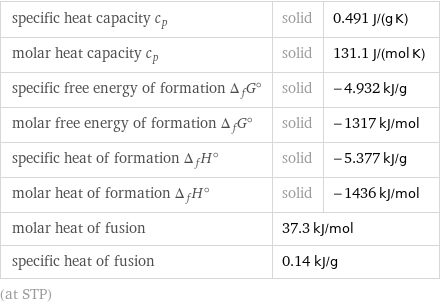
specific heat capacity c_p | solid | 0.491 J/(g K) molar heat capacity c_p | solid | 131.1 J/(mol K) specific free energy of formation Δ_fG° | solid | -4.932 kJ/g molar free energy of formation Δ_fG° | solid | -1317 kJ/mol specific heat of formation Δ_fH° | solid | -5.377 kJ/g molar heat of formation Δ_fH° | solid | -1436 kJ/mol molar heat of fusion | 37.3 kJ/mol | specific heat of fusion | 0.14 kJ/g | (at STP)
Chemical identifiers
![CAS number | 7488-54-2 SMILES identifier | O=S(=O)([O-])[O-].[Rb+].[Rb+] RTECS number | WS8350000 MDL number | MFCD00011190](../image_source/24aa5b785a0a91bc6a2d566fbcc2d2aa.png)
CAS number | 7488-54-2 SMILES identifier | O=S(=O)([O-])[O-].[Rb+].[Rb+] RTECS number | WS8350000 MDL number | MFCD00011190