Input interpretation

sodium perfluoroacetate | element types
Result
carbon | fluorine | sodium | oxygen
Periodic table location
Periodic table location
Images

Images
Basic elemental properties
![| atomic symbol | atomic number | short electronic configuration carbon | C | 6 | [He]2s^22p^2 fluorine | F | 9 | [He]2s^22p^5 sodium | Na | 11 | [Ne]3s^1 oxygen | O | 8 | [He]2s^22p^4 | Aufbau diagram | block | group carbon | 2p 2s | p | 14 fluorine | 2p 2s | p | 17 sodium | 3s | s | 1 oxygen | 2p 2s | p | 16 | period | atomic mass | half-life carbon | 2 | 12.011 u | (stable) fluorine | 2 | 18.998403163 u | (stable) sodium | 3 | 22.98976928 u | (stable) oxygen | 2 | 15.999 u | (stable)](../image_source/05dc939b2232273d91d52d8beff4945d.png)
| atomic symbol | atomic number | short electronic configuration carbon | C | 6 | [He]2s^22p^2 fluorine | F | 9 | [He]2s^22p^5 sodium | Na | 11 | [Ne]3s^1 oxygen | O | 8 | [He]2s^22p^4 | Aufbau diagram | block | group carbon | 2p 2s | p | 14 fluorine | 2p 2s | p | 17 sodium | 3s | s | 1 oxygen | 2p 2s | p | 16 | period | atomic mass | half-life carbon | 2 | 12.011 u | (stable) fluorine | 2 | 18.998403163 u | (stable) sodium | 3 | 22.98976928 u | (stable) oxygen | 2 | 15.999 u | (stable)
Thermodynamic properties
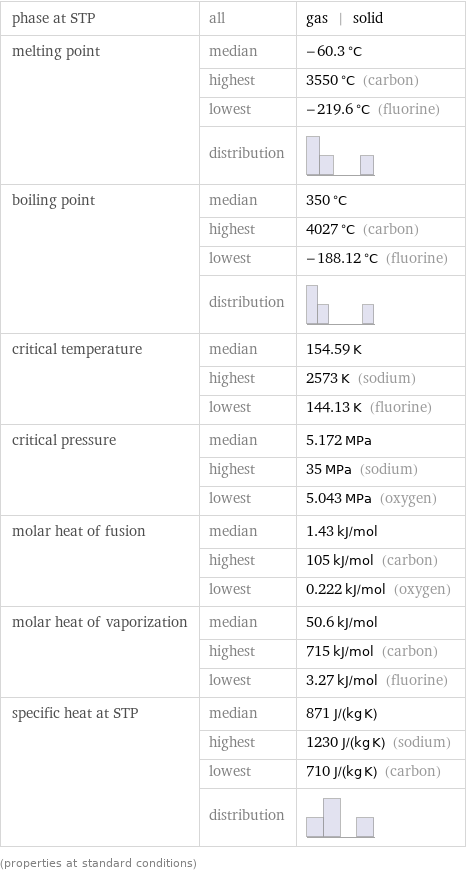
phase at STP | all | gas | solid melting point | median | -60.3 °C | highest | 3550 °C (carbon) | lowest | -219.6 °C (fluorine) | distribution | boiling point | median | 350 °C | highest | 4027 °C (carbon) | lowest | -188.12 °C (fluorine) | distribution | critical temperature | median | 154.59 K | highest | 2573 K (sodium) | lowest | 144.13 K (fluorine) critical pressure | median | 5.172 MPa | highest | 35 MPa (sodium) | lowest | 5.043 MPa (oxygen) molar heat of fusion | median | 1.43 kJ/mol | highest | 105 kJ/mol (carbon) | lowest | 0.222 kJ/mol (oxygen) molar heat of vaporization | median | 50.6 kJ/mol | highest | 715 kJ/mol (carbon) | lowest | 3.27 kJ/mol (fluorine) specific heat at STP | median | 871 J/(kg K) | highest | 1230 J/(kg K) (sodium) | lowest | 710 J/(kg K) (carbon) | distribution | (properties at standard conditions)
Material properties
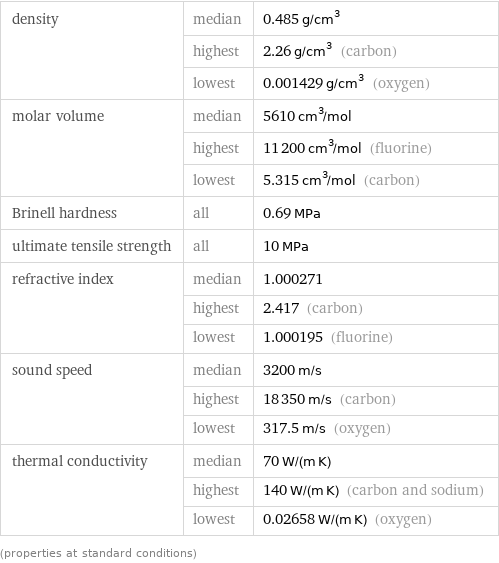
density | median | 0.485 g/cm^3 | highest | 2.26 g/cm^3 (carbon) | lowest | 0.001429 g/cm^3 (oxygen) molar volume | median | 5610 cm^3/mol | highest | 11200 cm^3/mol (fluorine) | lowest | 5.315 cm^3/mol (carbon) Brinell hardness | all | 0.69 MPa ultimate tensile strength | all | 10 MPa refractive index | median | 1.000271 | highest | 2.417 (carbon) | lowest | 1.000195 (fluorine) sound speed | median | 3200 m/s | highest | 18350 m/s (carbon) | lowest | 317.5 m/s (oxygen) thermal conductivity | median | 70 W/(m K) | highest | 140 W/(m K) (carbon and sodium) | lowest | 0.02658 W/(m K) (oxygen) (properties at standard conditions)
Reactivity
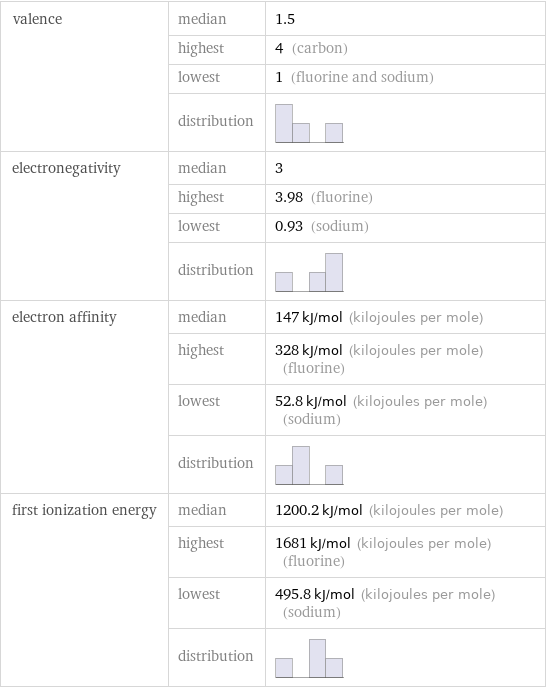
valence | median | 1.5 | highest | 4 (carbon) | lowest | 1 (fluorine and sodium) | distribution | electronegativity | median | 3 | highest | 3.98 (fluorine) | lowest | 0.93 (sodium) | distribution | electron affinity | median | 147 kJ/mol (kilojoules per mole) | highest | 328 kJ/mol (kilojoules per mole) (fluorine) | lowest | 52.8 kJ/mol (kilojoules per mole) (sodium) | distribution | first ionization energy | median | 1200.2 kJ/mol (kilojoules per mole) | highest | 1681 kJ/mol (kilojoules per mole) (fluorine) | lowest | 495.8 kJ/mol (kilojoules per mole) (sodium) | distribution |
Atomic properties
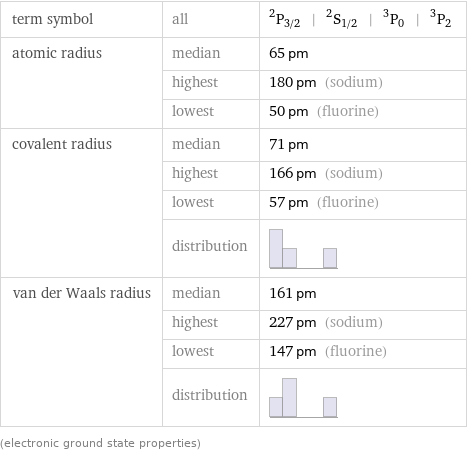
term symbol | all | ^2P_(3/2) | ^2S_(1/2) | ^3P_0 | ^3P_2 atomic radius | median | 65 pm | highest | 180 pm (sodium) | lowest | 50 pm (fluorine) covalent radius | median | 71 pm | highest | 166 pm (sodium) | lowest | 57 pm (fluorine) | distribution | van der Waals radius | median | 161 pm | highest | 227 pm (sodium) | lowest | 147 pm (fluorine) | distribution | (electronic ground state properties)
Abundances
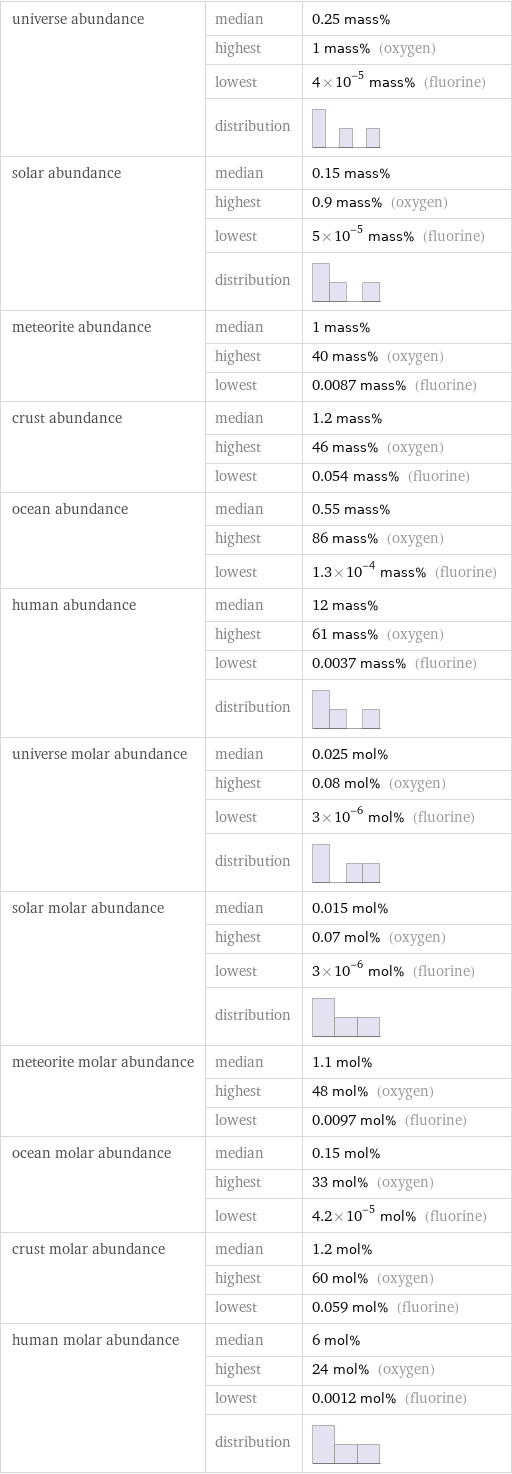
universe abundance | median | 0.25 mass% | highest | 1 mass% (oxygen) | lowest | 4×10^-5 mass% (fluorine) | distribution | solar abundance | median | 0.15 mass% | highest | 0.9 mass% (oxygen) | lowest | 5×10^-5 mass% (fluorine) | distribution | meteorite abundance | median | 1 mass% | highest | 40 mass% (oxygen) | lowest | 0.0087 mass% (fluorine) crust abundance | median | 1.2 mass% | highest | 46 mass% (oxygen) | lowest | 0.054 mass% (fluorine) ocean abundance | median | 0.55 mass% | highest | 86 mass% (oxygen) | lowest | 1.3×10^-4 mass% (fluorine) human abundance | median | 12 mass% | highest | 61 mass% (oxygen) | lowest | 0.0037 mass% (fluorine) | distribution | universe molar abundance | median | 0.025 mol% | highest | 0.08 mol% (oxygen) | lowest | 3×10^-6 mol% (fluorine) | distribution | solar molar abundance | median | 0.015 mol% | highest | 0.07 mol% (oxygen) | lowest | 3×10^-6 mol% (fluorine) | distribution | meteorite molar abundance | median | 1.1 mol% | highest | 48 mol% (oxygen) | lowest | 0.0097 mol% (fluorine) ocean molar abundance | median | 0.15 mol% | highest | 33 mol% (oxygen) | lowest | 4.2×10^-5 mol% (fluorine) crust molar abundance | median | 1.2 mol% | highest | 60 mol% (oxygen) | lowest | 0.059 mol% (fluorine) human molar abundance | median | 6 mol% | highest | 24 mol% (oxygen) | lowest | 0.0012 mol% (fluorine) | distribution |