Input interpretation

lead(II) hexafluorosilicate dihydrate | barium perchlorate hydrate
Chemical names and formulas
| lead(II) hexafluorosilicate dihydrate | barium perchlorate hydrate formula | PbSiF_6·2H_2O | Ba(ClO_4)_2·xH_2O Hill formula | F_6H_4O_2PbSi | BaCl_2H_2O_9 name | lead(II) hexafluorosilicate dihydrate | barium perchlorate hydrate IUPAC name | | barium(+2) cation diperchlorate hydrate alternate names | (none) | barium(+2) cation diperchlorate hydrate mass fractions | F (fluorine) 29.6% | H (hydrogen) 1.05% | O (oxygen) 8.3% | Pb (lead) 53.8% | Si (silicon) 7.29% | Ba (barium) 38.8% | Cl (chlorine) 20% | H (hydrogen) 0.569% | O (oxygen) 40.6%
Structure diagrams
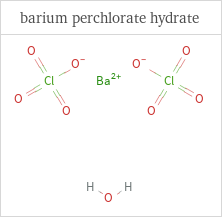
Structure diagrams
Basic properties

| lead(II) hexafluorosilicate dihydrate | barium perchlorate hydrate molar mass | 385.3 g/mol | 354.23 g/mol solubility in water | soluble |
Units
