Input interpretation
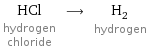
HCl hydrogen chloride ⟶ H_2 hydrogen
Sample reactions
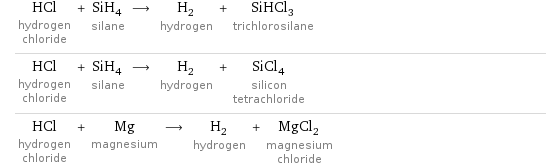
HCl hydrogen chloride + SiH_4 silane ⟶ H_2 hydrogen + SiHCl_3 trichlorosilane HCl hydrogen chloride + SiH_4 silane ⟶ H_2 hydrogen + SiCl_4 silicon tetrachloride HCl hydrogen chloride + Mg magnesium ⟶ H_2 hydrogen + MgCl_2 magnesium chloride
Chemical names and formulas
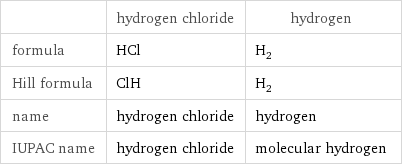
| hydrogen chloride | hydrogen formula | HCl | H_2 Hill formula | ClH | H_2 name | hydrogen chloride | hydrogen IUPAC name | hydrogen chloride | molecular hydrogen
Substance properties
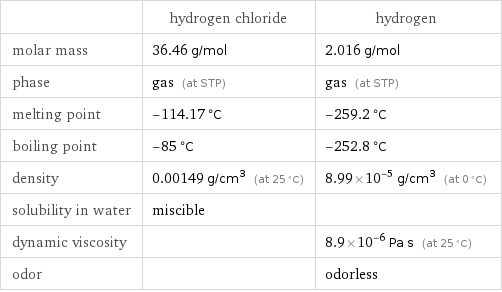
| hydrogen chloride | hydrogen molar mass | 36.46 g/mol | 2.016 g/mol phase | gas (at STP) | gas (at STP) melting point | -114.17 °C | -259.2 °C boiling point | -85 °C | -252.8 °C density | 0.00149 g/cm^3 (at 25 °C) | 8.99×10^-5 g/cm^3 (at 0 °C) solubility in water | miscible | dynamic viscosity | | 8.9×10^-6 Pa s (at 25 °C) odor | | odorless
Units
