Input interpretation
mercuric oxide
Chemical names and formulas
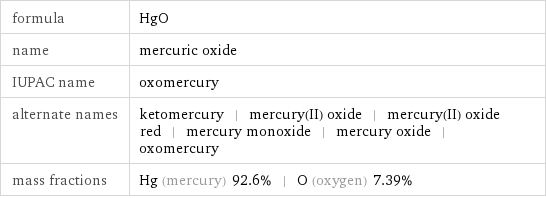
formula | HgO name | mercuric oxide IUPAC name | oxomercury alternate names | ketomercury | mercury(II) oxide | mercury(II) oxide red | mercury monoxide | mercury oxide | oxomercury mass fractions | Hg (mercury) 92.6% | O (oxygen) 7.39%
Structure diagram

Structure diagram

| bond counts | 1 bond
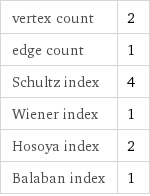
vertex count | 2 edge count | 1 Schultz index | 4 Wiener index | 1 Hosoya index | 2 Balaban index | 1
Basic properties
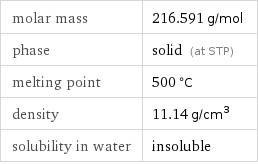
molar mass | 216.591 g/mol phase | solid (at STP) melting point | 500 °C density | 11.14 g/cm^3 solubility in water | insoluble
Units

Solid properties (at STP)
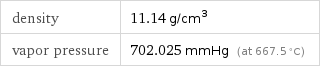
density | 11.14 g/cm^3 vapor pressure | 702.025 mmHg (at 667.5 °C)
Units

Thermodynamic properties
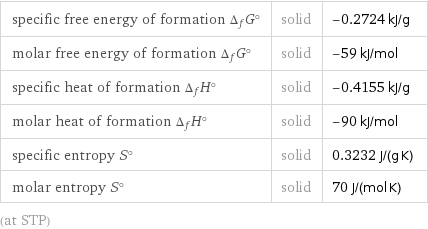
specific free energy of formation Δ_fG° | solid | -0.2724 kJ/g molar free energy of formation Δ_fG° | solid | -59 kJ/mol specific heat of formation Δ_fH° | solid | -0.4155 kJ/g molar heat of formation Δ_fH° | solid | -90 kJ/mol specific entropy S° | solid | 0.3232 J/(g K) molar entropy S° | solid | 70 J/(mol K) (at STP)
Chemical identifiers
![CAS number | 21908-53-2 PubChem CID number | 30856 PubChem SID number | 24852200 SMILES identifier | O=[Hg] InChI identifier | InChI=1/Hg.O/rHgO/c1-2 RTECS number | OW8750000 MDL number | MFCD00011045](../image_source/83c68a36f0fdf4c070a47824b7af21e7.png)
CAS number | 21908-53-2 PubChem CID number | 30856 PubChem SID number | 24852200 SMILES identifier | O=[Hg] InChI identifier | InChI=1/Hg.O/rHgO/c1-2 RTECS number | OW8750000 MDL number | MFCD00011045
NFPA label

NFPA label

NFPA health rating | 3 NFPA fire rating | 0 NFPA reactivity rating | 0
Toxicity properties

odor | odorless lethal dosage | 18 mg/kg (oral dose for rats)

probable lethal dose for man | 4 mL (milliliters) RTECS classes | agricultural chemical and pesticide | tumorigen | reproductive effector