Input interpretation

alkali metals | phase at STP
Summary
solid
Table

lithium | solid sodium | solid potassium | solid rubidium | solid cesium | solid francium | solid
Thermodynamic properties
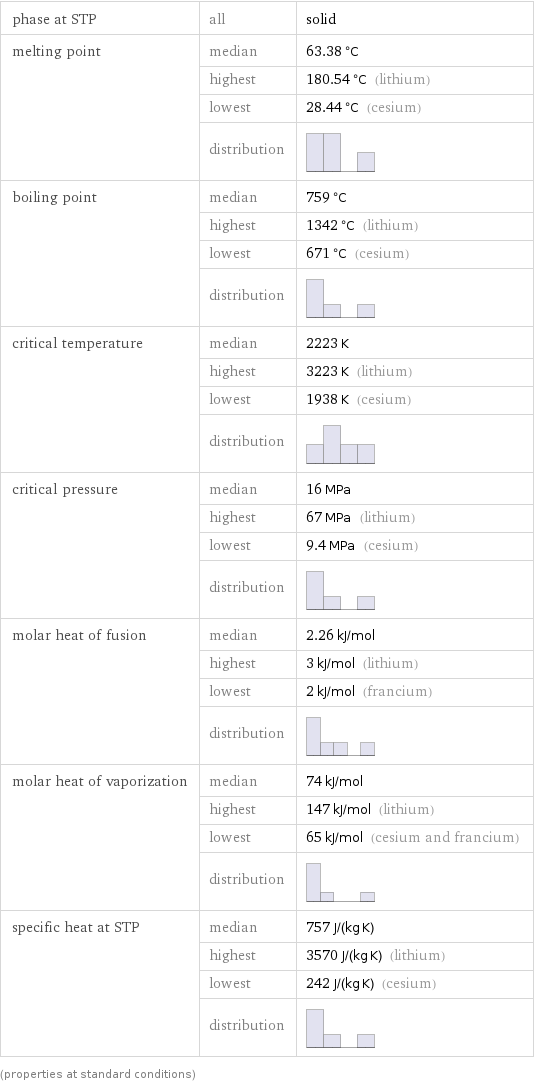
phase at STP | all | solid melting point | median | 63.38 °C | highest | 180.54 °C (lithium) | lowest | 28.44 °C (cesium) | distribution | boiling point | median | 759 °C | highest | 1342 °C (lithium) | lowest | 671 °C (cesium) | distribution | critical temperature | median | 2223 K | highest | 3223 K (lithium) | lowest | 1938 K (cesium) | distribution | critical pressure | median | 16 MPa | highest | 67 MPa (lithium) | lowest | 9.4 MPa (cesium) | distribution | molar heat of fusion | median | 2.26 kJ/mol | highest | 3 kJ/mol (lithium) | lowest | 2 kJ/mol (francium) | distribution | molar heat of vaporization | median | 74 kJ/mol | highest | 147 kJ/mol (lithium) | lowest | 65 kJ/mol (cesium and francium) | distribution | specific heat at STP | median | 757 J/(kg K) | highest | 3570 J/(kg K) (lithium) | lowest | 242 J/(kg K) (cesium) | distribution | (properties at standard conditions)