Input interpretation

organic solvents | molar heat of combustion
Summary
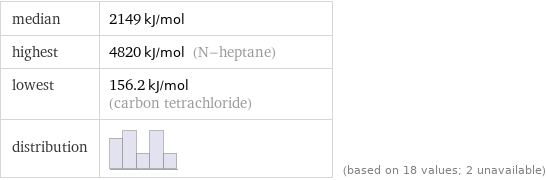
median | 2149 kJ/mol highest | 4820 kJ/mol (N-heptane) lowest | 156.2 kJ/mol (carbon tetrachloride) distribution | | (based on 18 values; 2 unavailable)
Entities with missing values
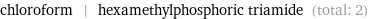
chloroform | hexamethylphosphoric triamide (total: 2)
Distribution plots
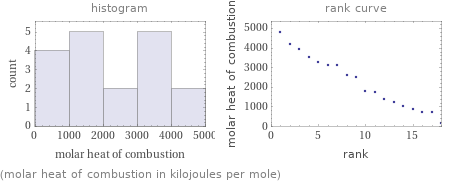
(molar heat of combustion in kilojoules per mole)
Molar heat of combustion rankings
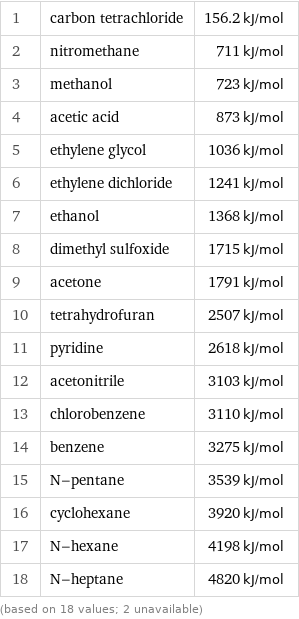
1 | carbon tetrachloride | 156.2 kJ/mol 2 | nitromethane | 711 kJ/mol 3 | methanol | 723 kJ/mol 4 | acetic acid | 873 kJ/mol 5 | ethylene glycol | 1036 kJ/mol 6 | ethylene dichloride | 1241 kJ/mol 7 | ethanol | 1368 kJ/mol 8 | dimethyl sulfoxide | 1715 kJ/mol 9 | acetone | 1791 kJ/mol 10 | tetrahydrofuran | 2507 kJ/mol 11 | pyridine | 2618 kJ/mol 12 | acetonitrile | 3103 kJ/mol 13 | chlorobenzene | 3110 kJ/mol 14 | benzene | 3275 kJ/mol 15 | N-pentane | 3539 kJ/mol 16 | cyclohexane | 3920 kJ/mol 17 | N-hexane | 4198 kJ/mol 18 | N-heptane | 4820 kJ/mol (based on 18 values; 2 unavailable)
Unit conversions for median molar heat of combustion 2149 kJ/mol
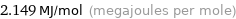
2.149 MJ/mol (megajoules per mole)
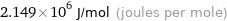
2.149×10^6 J/mol (joules per mole)
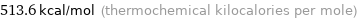
513.6 kcal/mol (thermochemical kilocalories per mole)

22.27 eV (molar electronvolts)