Input interpretation
graphite | gray antimony
Chemical names and formulas
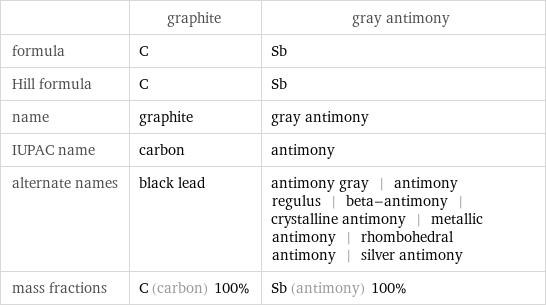
| graphite | gray antimony formula | C | Sb Hill formula | C | Sb name | graphite | gray antimony IUPAC name | carbon | antimony alternate names | black lead | antimony gray | antimony regulus | beta-antimony | crystalline antimony | metallic antimony | rhombohedral antimony | silver antimony mass fractions | C (carbon) 100% | Sb (antimony) 100%
Structure diagrams

Structure diagrams
Basic properties
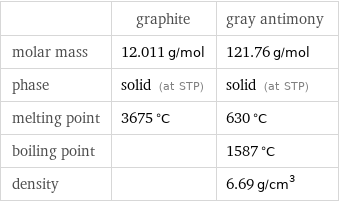
| graphite | gray antimony molar mass | 12.011 g/mol | 121.76 g/mol phase | solid (at STP) | solid (at STP) melting point | 3675 °C | 630 °C boiling point | | 1587 °C density | | 6.69 g/cm^3
Units

Thermodynamic properties

| graphite | gray antimony specific heat of vaporization | | 0.9256 kJ/g (kilojoules per gram) specific heat of fusion | 9.77 kJ/g (kilojoules per gram) | 0.1625 kJ/g (kilojoules per gram)
Solid properties (at STP)
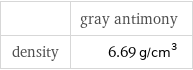
| gray antimony density | 6.69 g/cm^3
Units
