Input interpretation
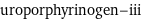
uroporphyrinogen-iii
Chemical names and formulas

formula | C_40H_44N_4O_16 name | uroporphyrinogen-iii mass fractions | C (carbon) 58% | H (hydrogen) 4.38% | N (nitrogen) 6.76% | O (oxygen) 30.9%
Lewis structure
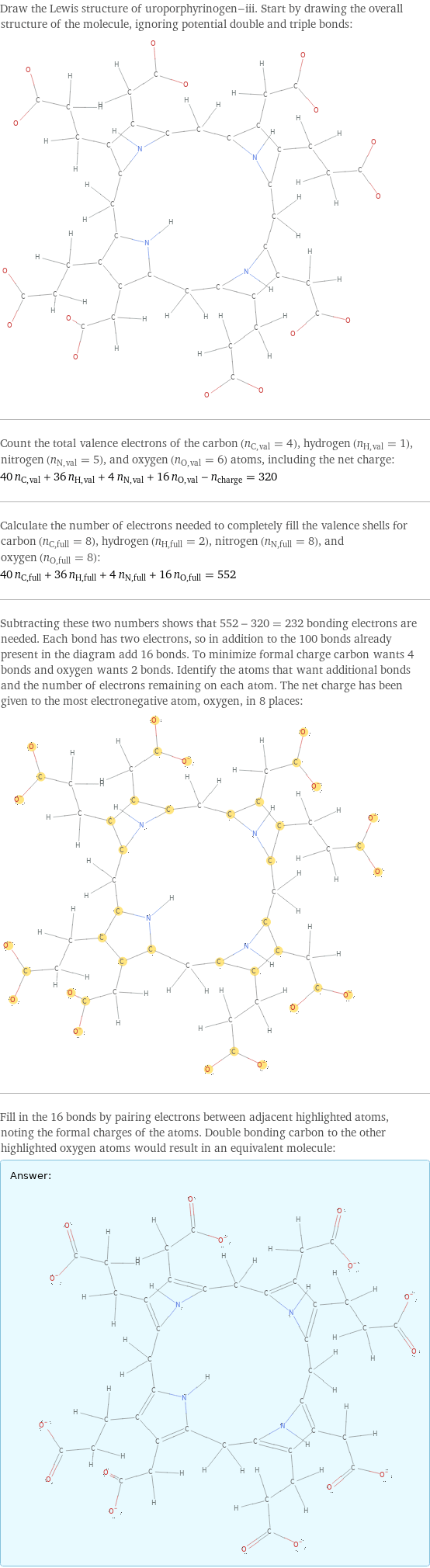
Draw the Lewis structure of uroporphyrinogen-iii. Start by drawing the overall structure of the molecule, ignoring potential double and triple bonds: Count the total valence electrons of the carbon (n_C, val = 4), hydrogen (n_H, val = 1), nitrogen (n_N, val = 5), and oxygen (n_O, val = 6) atoms, including the net charge: 40 n_C, val + 36 n_H, val + 4 n_N, val + 16 n_O, val - n_charge = 320 Calculate the number of electrons needed to completely fill the valence shells for carbon (n_C, full = 8), hydrogen (n_H, full = 2), nitrogen (n_N, full = 8), and oxygen (n_O, full = 8): 40 n_C, full + 36 n_H, full + 4 n_N, full + 16 n_O, full = 552 Subtracting these two numbers shows that 552 - 320 = 232 bonding electrons are needed. Each bond has two electrons, so in addition to the 100 bonds already present in the diagram add 16 bonds. To minimize formal charge carbon wants 4 bonds and oxygen wants 2 bonds. Identify the atoms that want additional bonds and the number of electrons remaining on each atom. The net charge has been given to the most electronegative atom, oxygen, in 8 places: Fill in the 16 bonds by pairing electrons between adjacent highlighted atoms, noting the formal charges of the atoms. Double bonding carbon to the other highlighted oxygen atoms would result in an equivalent molecule: Answer: | |
Basic properties

molar mass | 828.7 g/mol
Units

Chemical identifiers
![CAS number | 1976-85-8 PubChem CID number | 25202645 SMILES identifier | C(=O)([O-])CCC3(C(=C2(CC5(NC(CC4(NC(CC1(NC(=C(C=1CC(=O)[O-])CCC(=O)[O-])CC(N2)=3))=C(CC(=O)[O-])C=4CCC(=O)[O-]))=C(CC([O-])=O)C(CCC(=O)[O-])=5)))CC(=O)[O-])](../image_source/0792d93fb6f49e60477a7169240a6679.png)
CAS number | 1976-85-8 PubChem CID number | 25202645 SMILES identifier | C(=O)([O-])CCC3(C(=C2(CC5(NC(CC4(NC(CC1(NC(=C(C=1CC(=O)[O-])CCC(=O)[O-])CC(N2)=3))=C(CC(=O)[O-])C=4CCC(=O)[O-]))=C(CC([O-])=O)C(CCC(=O)[O-])=5)))CC(=O)[O-])