Input interpretation
ammonium iron(III) oxalate trihydrate | stannous sulfate
Chemical names and formulas

| ammonium iron(III) oxalate trihydrate | stannous sulfate formula | (NH_4)_3Fe(C_2O_4)_3·3H_2O | SnSO_4 Hill formula | C_6FeH_18N_3O_15 | O_4SSn name | ammonium iron(III) oxalate trihydrate | stannous sulfate IUPAC name | | tin(+2) cation sulfate alternate names | (none) | stannous sulphate | tin(2+) sulfate | tin(II) sulfate | tin(II) sulphate mass fractions | C (carbon) 15.8% | Fe (iron) 18.4% | H (hydrogen) 3.32% | N (nitrogen) 4.61% | O (oxygen) 57.9% | O (oxygen) 29.8% | S (sulfur) 14.9% | Sn (tin) 55.3%
Structure diagrams
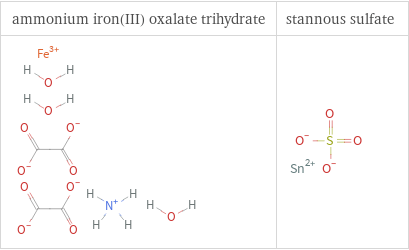
Structure diagrams
Basic properties
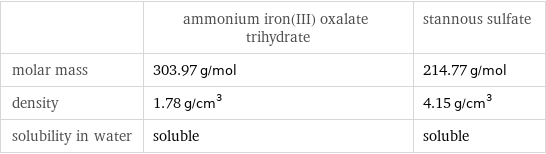
| ammonium iron(III) oxalate trihydrate | stannous sulfate molar mass | 303.97 g/mol | 214.77 g/mol density | 1.78 g/cm^3 | 4.15 g/cm^3 solubility in water | soluble | soluble
Units
