Input interpretation

gaseous elements (at STP) | atomic mass
Summary
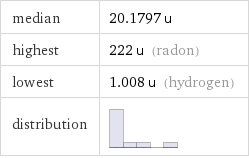
median | 20.1797 u highest | 222 u (radon) lowest | 1.008 u (hydrogen) distribution |
Units

Distribution plots
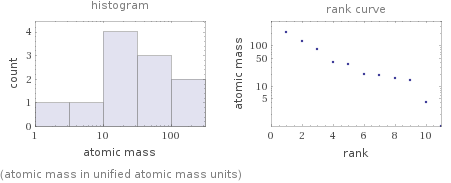
(atomic mass in unified atomic mass units)
Atomic mass rankings
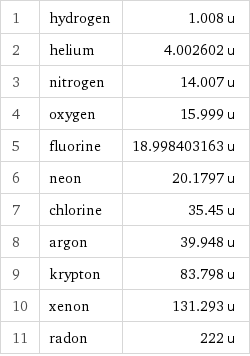
1 | hydrogen | 1.008 u 2 | helium | 4.002602 u 3 | nitrogen | 14.007 u 4 | oxygen | 15.999 u 5 | fluorine | 18.998403163 u 6 | neon | 20.1797 u 7 | chlorine | 35.45 u 8 | argon | 39.948 u 9 | krypton | 83.798 u 10 | xenon | 131.293 u 11 | radon | 222 u
Unit conversions for median atomic mass 20.1797 u
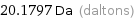
20.1797 Da (daltons)
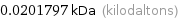
0.0201797 kDa (kilodaltons)
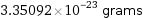
3.35092×10^-23 grams
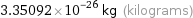
3.35092×10^-26 kg (kilograms)
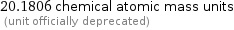
20.1806 chemical atomic mass units (unit officially deprecated)
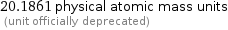
20.1861 physical atomic mass units (unit officially deprecated)
Corresponding quantities
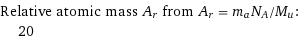
Relative atomic mass A_r from A_r = m_aN_A/M_u: | 20
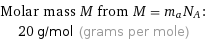
Molar mass M from M = m_aN_A: | 20 g/mol (grams per mole)