Input interpretation
calcium hydroxide | barium hydroxide
Chemical names and formulas
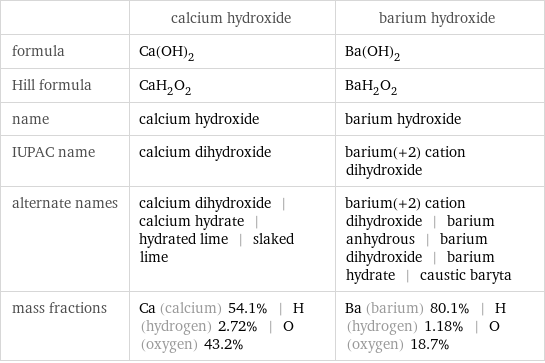
| calcium hydroxide | barium hydroxide formula | Ca(OH)_2 | Ba(OH)_2 Hill formula | CaH_2O_2 | BaH_2O_2 name | calcium hydroxide | barium hydroxide IUPAC name | calcium dihydroxide | barium(+2) cation dihydroxide alternate names | calcium dihydroxide | calcium hydrate | hydrated lime | slaked lime | barium(+2) cation dihydroxide | barium anhydrous | barium dihydroxide | barium hydrate | caustic baryta mass fractions | Ca (calcium) 54.1% | H (hydrogen) 2.72% | O (oxygen) 43.2% | Ba (barium) 80.1% | H (hydrogen) 1.18% | O (oxygen) 18.7%
Structure diagrams
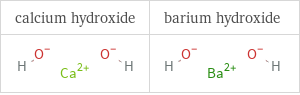
Structure diagrams
Basic properties
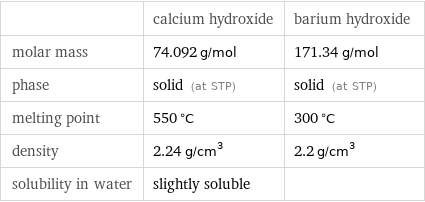
| calcium hydroxide | barium hydroxide molar mass | 74.092 g/mol | 171.34 g/mol phase | solid (at STP) | solid (at STP) melting point | 550 °C | 300 °C density | 2.24 g/cm^3 | 2.2 g/cm^3 solubility in water | slightly soluble |
Units

Solid properties (at STP)

| calcium hydroxide | barium hydroxide density | 2.24 g/cm^3 | 2.2 g/cm^3 refractive index | 1.555 |
Units

Chemical identifiers
![| calcium hydroxide | barium hydroxide CAS number | 1305-62-0 | 17194-00-2 PubChem CID number | 14777 | 28387 PubChem SID number | 24868536 | 24867139 SMILES identifier | [OH-].[OH-].[Ca+2] | [OH-].[OH-].[Ba+2]](../image_source/4117fcddfbdcb890b72296e11925952a.png)
| calcium hydroxide | barium hydroxide CAS number | 1305-62-0 | 17194-00-2 PubChem CID number | 14777 | 28387 PubChem SID number | 24868536 | 24867139 SMILES identifier | [OH-].[OH-].[Ca+2] | [OH-].[OH-].[Ba+2]