Input interpretation
lead(II) chlorate | zinc oxide hydrate
Chemical names and formulas
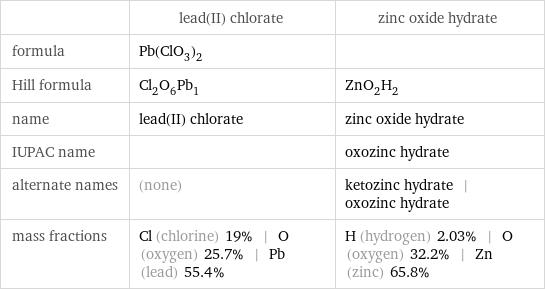
| lead(II) chlorate | zinc oxide hydrate formula | Pb(ClO_3)_2 | Hill formula | Cl_2O_6Pb_1 | ZnO_2H_2 name | lead(II) chlorate | zinc oxide hydrate IUPAC name | | oxozinc hydrate alternate names | (none) | ketozinc hydrate | oxozinc hydrate mass fractions | Cl (chlorine) 19% | O (oxygen) 25.7% | Pb (lead) 55.4% | H (hydrogen) 2.03% | O (oxygen) 32.2% | Zn (zinc) 65.8%
Structure diagrams
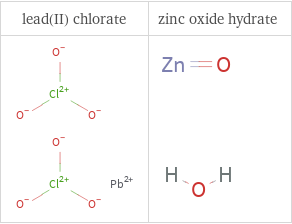
Structure diagrams
Basic properties
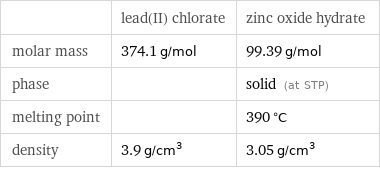
| lead(II) chlorate | zinc oxide hydrate molar mass | 374.1 g/mol | 99.39 g/mol phase | | solid (at STP) melting point | | 390 °C density | 3.9 g/cm^3 | 3.05 g/cm^3
Units
