Input interpretation
sodium hydride | rubidium fluoride
Chemical names and formulas
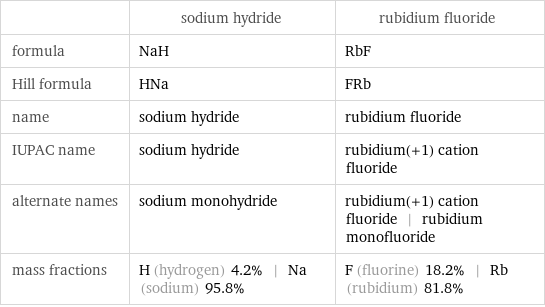
| sodium hydride | rubidium fluoride formula | NaH | RbF Hill formula | HNa | FRb name | sodium hydride | rubidium fluoride IUPAC name | sodium hydride | rubidium(+1) cation fluoride alternate names | sodium monohydride | rubidium(+1) cation fluoride | rubidium monofluoride mass fractions | H (hydrogen) 4.2% | Na (sodium) 95.8% | F (fluorine) 18.2% | Rb (rubidium) 81.8%
Structure diagrams

Structure diagrams
Basic properties
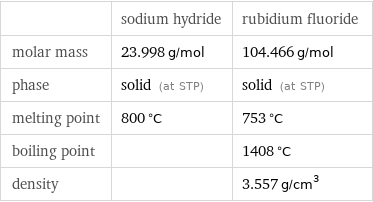
| sodium hydride | rubidium fluoride molar mass | 23.998 g/mol | 104.466 g/mol phase | solid (at STP) | solid (at STP) melting point | 800 °C | 753 °C boiling point | | 1408 °C density | | 3.557 g/cm^3
Units

Thermodynamic properties

| sodium hydride | rubidium fluoride specific heat of fusion | 1.1 kJ/g (kilojoules per gram) | 0.247 kJ/g (kilojoules per gram)