Input interpretation
sodium fluoride | sodium bromate
Chemical names and formulas
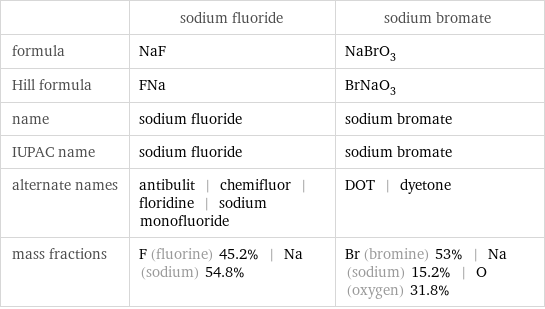
| sodium fluoride | sodium bromate formula | NaF | NaBrO_3 Hill formula | FNa | BrNaO_3 name | sodium fluoride | sodium bromate IUPAC name | sodium fluoride | sodium bromate alternate names | antibulit | chemifluor | floridine | sodium monofluoride | DOT | dyetone mass fractions | F (fluorine) 45.2% | Na (sodium) 54.8% | Br (bromine) 53% | Na (sodium) 15.2% | O (oxygen) 31.8%
Structure diagrams
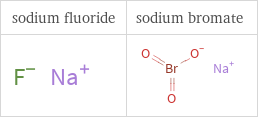
Structure diagrams
Basic properties
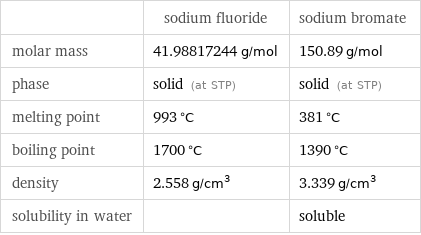
| sodium fluoride | sodium bromate molar mass | 41.98817244 g/mol | 150.89 g/mol phase | solid (at STP) | solid (at STP) melting point | 993 °C | 381 °C boiling point | 1700 °C | 1390 °C density | 2.558 g/cm^3 | 3.339 g/cm^3 solubility in water | | soluble
Units
