Input interpretation

solid elements (at STP) | atomic mass
Summary
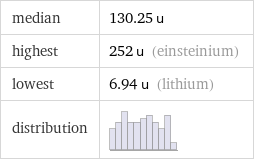
median | 130.25 u highest | 252 u (einsteinium) lowest | 6.94 u (lithium) distribution |
Units

Distribution plots
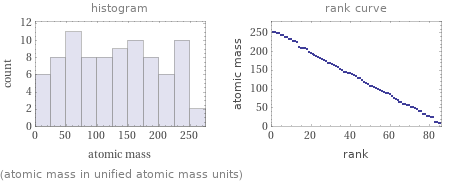
(atomic mass in unified atomic mass units)
Atomic mass rankings
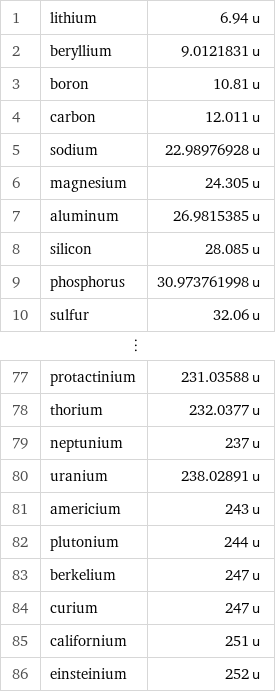
1 | lithium | 6.94 u 2 | beryllium | 9.0121831 u 3 | boron | 10.81 u 4 | carbon | 12.011 u 5 | sodium | 22.98976928 u 6 | magnesium | 24.305 u 7 | aluminum | 26.9815385 u 8 | silicon | 28.085 u 9 | phosphorus | 30.973761998 u 10 | sulfur | 32.06 u ⋮ | | 77 | protactinium | 231.03588 u 78 | thorium | 232.0377 u 79 | neptunium | 237 u 80 | uranium | 238.02891 u 81 | americium | 243 u 82 | plutonium | 244 u 83 | berkelium | 247 u 84 | curium | 247 u 85 | californium | 251 u 86 | einsteinium | 252 u
Unit conversions for median atomic mass 130.25 u
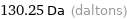
130.25 Da (daltons)
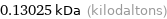
0.13025 kDa (kilodaltons)
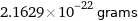
2.1629×10^-22 grams
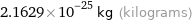
2.1629×10^-25 kg (kilograms)

130.26 chemical atomic mass units (unit officially deprecated)
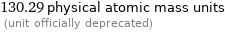
130.29 physical atomic mass units (unit officially deprecated)
Corresponding quantities
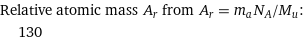
Relative atomic mass A_r from A_r = m_aN_A/M_u: | 130
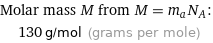
Molar mass M from M = m_aN_A: | 130 g/mol (grams per mole)