Input interpretation

germanium (chemical element) | arsenic (chemical element) | chlorine (chemical element)
Periodic table location
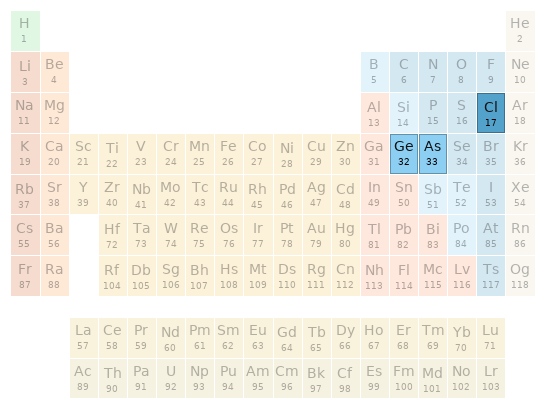
Periodic table location
Images
Images
Basic elemental properties
![| germanium | arsenic | chlorine atomic symbol | Ge | As | Cl atomic number | 32 | 33 | 17 short electronic configuration | [Ar]4s^23d^104p^2 | [Ar]4s^23d^104p^3 | [Ne]3s^23p^5 Aufbau diagram | 4p 3d 4s | 4p 3d 4s | 3p 3s block | p | p | p group | 14 | 15 | 17 period | 4 | 4 | 3 atomic mass | 72.63 u | 74.921595 u | 35.45 u half-life | (stable) | (stable) | (stable)](../image_source/8dc04d7e86cb5ba290a3485a42c8ff10.png)
| germanium | arsenic | chlorine atomic symbol | Ge | As | Cl atomic number | 32 | 33 | 17 short electronic configuration | [Ar]4s^23d^104p^2 | [Ar]4s^23d^104p^3 | [Ne]3s^23p^5 Aufbau diagram | 4p 3d 4s | 4p 3d 4s | 3p 3s block | p | p | p group | 14 | 15 | 17 period | 4 | 4 | 3 atomic mass | 72.63 u | 74.921595 u | 35.45 u half-life | (stable) | (stable) | (stable)
Thermodynamic properties
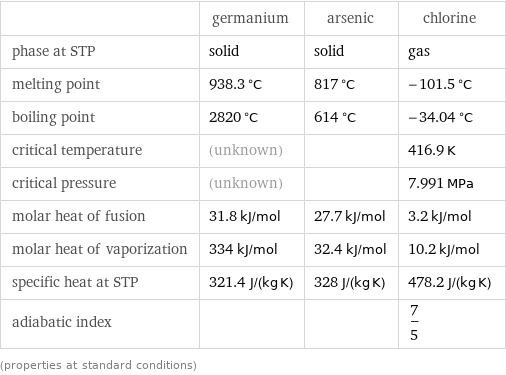
| germanium | arsenic | chlorine phase at STP | solid | solid | gas melting point | 938.3 °C | 817 °C | -101.5 °C boiling point | 2820 °C | 614 °C | -34.04 °C critical temperature | (unknown) | | 416.9 K critical pressure | (unknown) | | 7.991 MPa molar heat of fusion | 31.8 kJ/mol | 27.7 kJ/mol | 3.2 kJ/mol molar heat of vaporization | 334 kJ/mol | 32.4 kJ/mol | 10.2 kJ/mol specific heat at STP | 321.4 J/(kg K) | 328 J/(kg K) | 478.2 J/(kg K) adiabatic index | | | 7/5 (properties at standard conditions)
Material properties
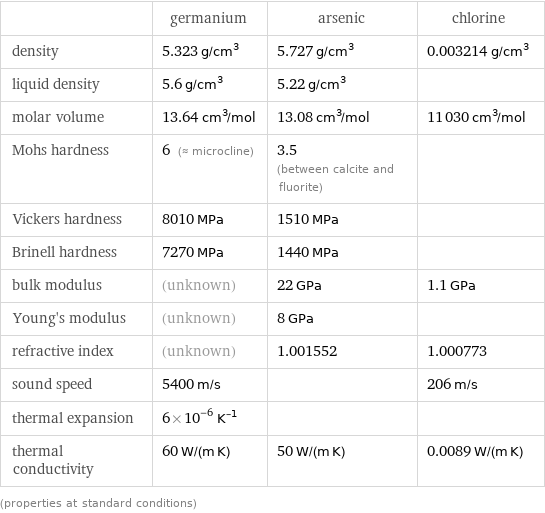
| germanium | arsenic | chlorine density | 5.323 g/cm^3 | 5.727 g/cm^3 | 0.003214 g/cm^3 liquid density | 5.6 g/cm^3 | 5.22 g/cm^3 | molar volume | 13.64 cm^3/mol | 13.08 cm^3/mol | 11030 cm^3/mol Mohs hardness | 6 (≈ microcline) | 3.5 (between calcite and fluorite) | Vickers hardness | 8010 MPa | 1510 MPa | Brinell hardness | 7270 MPa | 1440 MPa | bulk modulus | (unknown) | 22 GPa | 1.1 GPa Young's modulus | (unknown) | 8 GPa | refractive index | (unknown) | 1.001552 | 1.000773 sound speed | 5400 m/s | | 206 m/s thermal expansion | 6×10^-6 K^(-1) | | thermal conductivity | 60 W/(m K) | 50 W/(m K) | 0.0089 W/(m K) (properties at standard conditions)
Electromagnetic properties
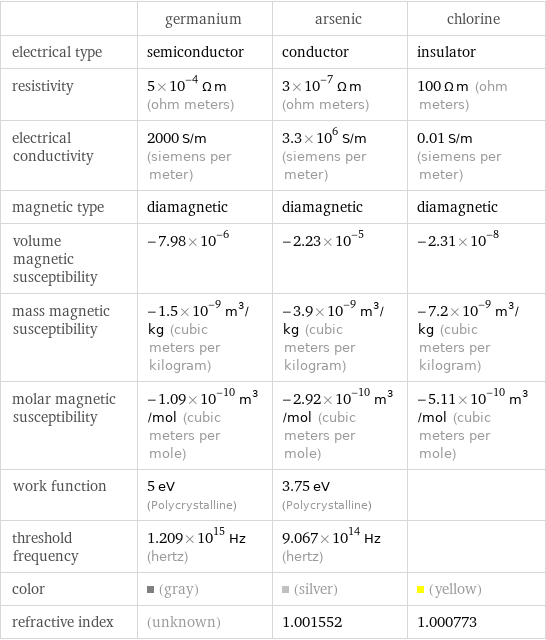
| germanium | arsenic | chlorine electrical type | semiconductor | conductor | insulator resistivity | 5×10^-4 Ω m (ohm meters) | 3×10^-7 Ω m (ohm meters) | 100 Ω m (ohm meters) electrical conductivity | 2000 S/m (siemens per meter) | 3.3×10^6 S/m (siemens per meter) | 0.01 S/m (siemens per meter) magnetic type | diamagnetic | diamagnetic | diamagnetic volume magnetic susceptibility | -7.98×10^-6 | -2.23×10^-5 | -2.31×10^-8 mass magnetic susceptibility | -1.5×10^-9 m^3/kg (cubic meters per kilogram) | -3.9×10^-9 m^3/kg (cubic meters per kilogram) | -7.2×10^-9 m^3/kg (cubic meters per kilogram) molar magnetic susceptibility | -1.09×10^-10 m^3/mol (cubic meters per mole) | -2.92×10^-10 m^3/mol (cubic meters per mole) | -5.11×10^-10 m^3/mol (cubic meters per mole) work function | 5 eV (Polycrystalline) | 3.75 eV (Polycrystalline) | threshold frequency | 1.209×10^15 Hz (hertz) | 9.067×10^14 Hz (hertz) | color | (gray) | (silver) | (yellow) refractive index | (unknown) | 1.001552 | 1.000773
Reactivity
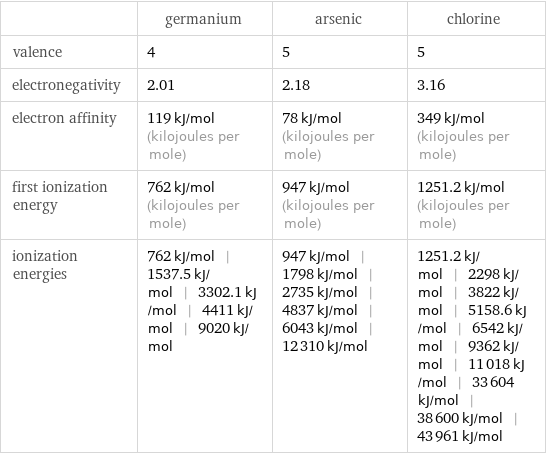
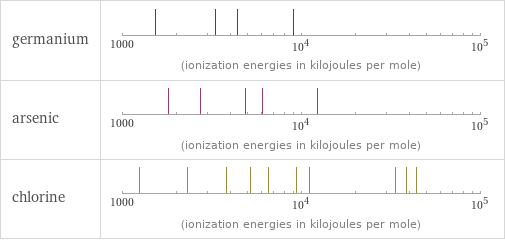
| germanium | arsenic | chlorine valence | 4 | 5 | 5 electronegativity | 2.01 | 2.18 | 3.16 electron affinity | 119 kJ/mol (kilojoules per mole) | 78 kJ/mol (kilojoules per mole) | 349 kJ/mol (kilojoules per mole) first ionization energy | 762 kJ/mol (kilojoules per mole) | 947 kJ/mol (kilojoules per mole) | 1251.2 kJ/mol (kilojoules per mole) ionization energies | 762 kJ/mol | 1537.5 kJ/mol | 3302.1 kJ/mol | 4411 kJ/mol | 9020 kJ/mol | 947 kJ/mol | 1798 kJ/mol | 2735 kJ/mol | 4837 kJ/mol | 6043 kJ/mol | 12310 kJ/mol | 1251.2 kJ/mol | 2298 kJ/mol | 3822 kJ/mol | 5158.6 kJ/mol | 6542 kJ/mol | 9362 kJ/mol | 11018 kJ/mol | 33604 kJ/mol | 38600 kJ/mol | 43961 kJ/mol
Reactivity
Atomic properties
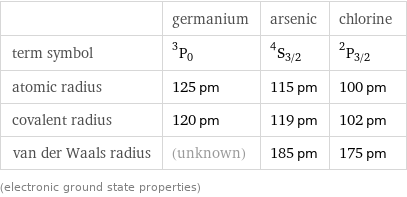
| germanium | arsenic | chlorine term symbol | ^3P_0 | ^4S_(3/2) | ^2P_(3/2) atomic radius | 125 pm | 115 pm | 100 pm covalent radius | 120 pm | 119 pm | 102 pm van der Waals radius | (unknown) | 185 pm | 175 pm (electronic ground state properties)
Abundances
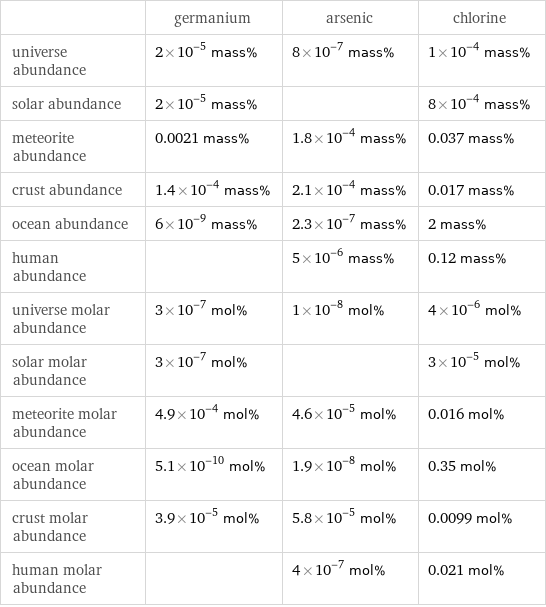
| germanium | arsenic | chlorine universe abundance | 2×10^-5 mass% | 8×10^-7 mass% | 1×10^-4 mass% solar abundance | 2×10^-5 mass% | | 8×10^-4 mass% meteorite abundance | 0.0021 mass% | 1.8×10^-4 mass% | 0.037 mass% crust abundance | 1.4×10^-4 mass% | 2.1×10^-4 mass% | 0.017 mass% ocean abundance | 6×10^-9 mass% | 2.3×10^-7 mass% | 2 mass% human abundance | | 5×10^-6 mass% | 0.12 mass% universe molar abundance | 3×10^-7 mol% | 1×10^-8 mol% | 4×10^-6 mol% solar molar abundance | 3×10^-7 mol% | | 3×10^-5 mol% meteorite molar abundance | 4.9×10^-4 mol% | 4.6×10^-5 mol% | 0.016 mol% ocean molar abundance | 5.1×10^-10 mol% | 1.9×10^-8 mol% | 0.35 mol% crust molar abundance | 3.9×10^-5 mol% | 5.8×10^-5 mol% | 0.0099 mol% human molar abundance | | 4×10^-7 mol% | 0.021 mol%