Input interpretation

hydrogen (chemical element) | bromine (chemical element) | oxygen (chemical element)
Periodic table location
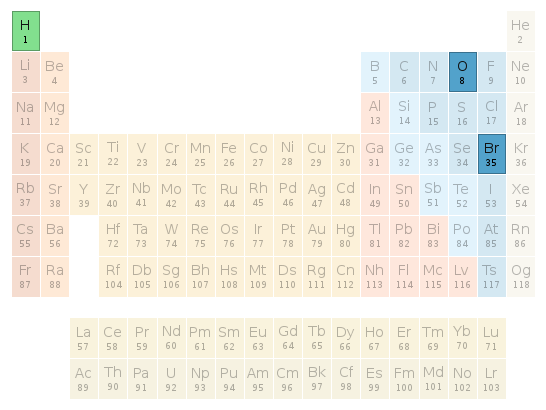
Periodic table location
Images
Images
Basic elemental properties
![| hydrogen | bromine | oxygen atomic symbol | H | Br | O atomic number | 1 | 35 | 8 short electronic configuration | 1s^1 | [Ar]4s^23d^104p^5 | [He]2s^22p^4 Aufbau diagram | 1s | 4p 3d 4s | 2p 2s block | s | p | p group | 1 | 17 | 16 period | 1 | 4 | 2 atomic mass | 1.008 u | 79.904 u | 15.999 u half-life | (stable) | (stable) | (stable)](../image_source/06dfbd8c864d0a4e13160133082a8247.png)
| hydrogen | bromine | oxygen atomic symbol | H | Br | O atomic number | 1 | 35 | 8 short electronic configuration | 1s^1 | [Ar]4s^23d^104p^5 | [He]2s^22p^4 Aufbau diagram | 1s | 4p 3d 4s | 2p 2s block | s | p | p group | 1 | 17 | 16 period | 1 | 4 | 2 atomic mass | 1.008 u | 79.904 u | 15.999 u half-life | (stable) | (stable) | (stable)
Thermodynamic properties
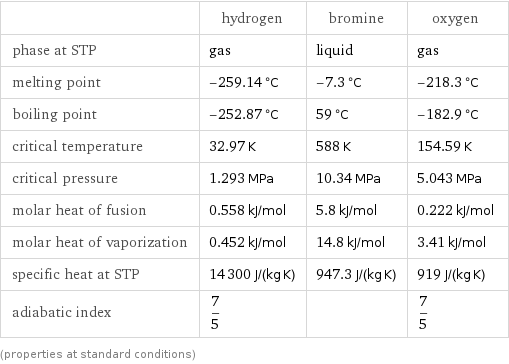
| hydrogen | bromine | oxygen phase at STP | gas | liquid | gas melting point | -259.14 °C | -7.3 °C | -218.3 °C boiling point | -252.87 °C | 59 °C | -182.9 °C critical temperature | 32.97 K | 588 K | 154.59 K critical pressure | 1.293 MPa | 10.34 MPa | 5.043 MPa molar heat of fusion | 0.558 kJ/mol | 5.8 kJ/mol | 0.222 kJ/mol molar heat of vaporization | 0.452 kJ/mol | 14.8 kJ/mol | 3.41 kJ/mol specific heat at STP | 14300 J/(kg K) | 947.3 J/(kg K) | 919 J/(kg K) adiabatic index | 7/5 | | 7/5 (properties at standard conditions)
Material properties
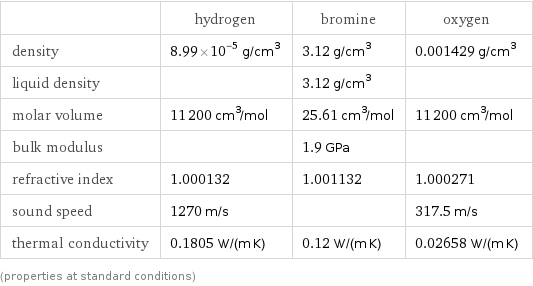
| hydrogen | bromine | oxygen density | 8.99×10^-5 g/cm^3 | 3.12 g/cm^3 | 0.001429 g/cm^3 liquid density | | 3.12 g/cm^3 | molar volume | 11200 cm^3/mol | 25.61 cm^3/mol | 11200 cm^3/mol bulk modulus | | 1.9 GPa | refractive index | 1.000132 | 1.001132 | 1.000271 sound speed | 1270 m/s | | 317.5 m/s thermal conductivity | 0.1805 W/(m K) | 0.12 W/(m K) | 0.02658 W/(m K) (properties at standard conditions)
Electromagnetic properties
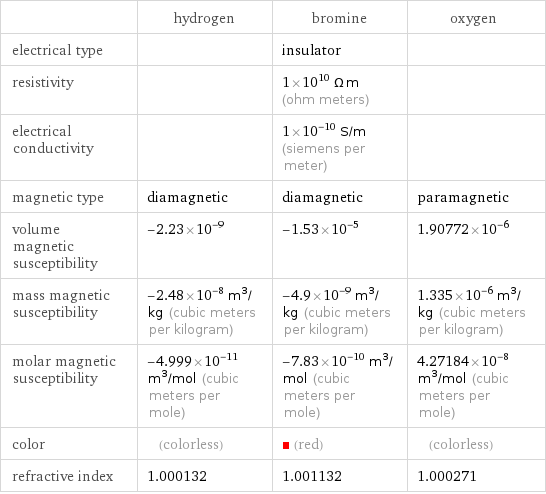
| hydrogen | bromine | oxygen electrical type | | insulator | resistivity | | 1×10^10 Ω m (ohm meters) | electrical conductivity | | 1×10^-10 S/m (siemens per meter) | magnetic type | diamagnetic | diamagnetic | paramagnetic volume magnetic susceptibility | -2.23×10^-9 | -1.53×10^-5 | 1.90772×10^-6 mass magnetic susceptibility | -2.48×10^-8 m^3/kg (cubic meters per kilogram) | -4.9×10^-9 m^3/kg (cubic meters per kilogram) | 1.335×10^-6 m^3/kg (cubic meters per kilogram) molar magnetic susceptibility | -4.999×10^-11 m^3/mol (cubic meters per mole) | -7.83×10^-10 m^3/mol (cubic meters per mole) | 4.27184×10^-8 m^3/mol (cubic meters per mole) color | (colorless) | (red) | (colorless) refractive index | 1.000132 | 1.001132 | 1.000271
Reactivity
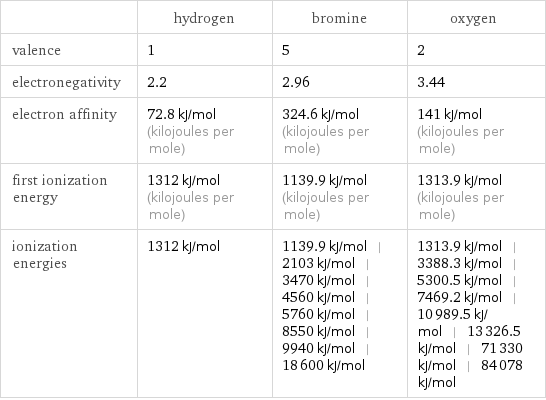
| hydrogen | bromine | oxygen valence | 1 | 5 | 2 electronegativity | 2.2 | 2.96 | 3.44 electron affinity | 72.8 kJ/mol (kilojoules per mole) | 324.6 kJ/mol (kilojoules per mole) | 141 kJ/mol (kilojoules per mole) first ionization energy | 1312 kJ/mol (kilojoules per mole) | 1139.9 kJ/mol (kilojoules per mole) | 1313.9 kJ/mol (kilojoules per mole) ionization energies | 1312 kJ/mol | 1139.9 kJ/mol | 2103 kJ/mol | 3470 kJ/mol | 4560 kJ/mol | 5760 kJ/mol | 8550 kJ/mol | 9940 kJ/mol | 18600 kJ/mol | 1313.9 kJ/mol | 3388.3 kJ/mol | 5300.5 kJ/mol | 7469.2 kJ/mol | 10989.5 kJ/mol | 13326.5 kJ/mol | 71330 kJ/mol | 84078 kJ/mol
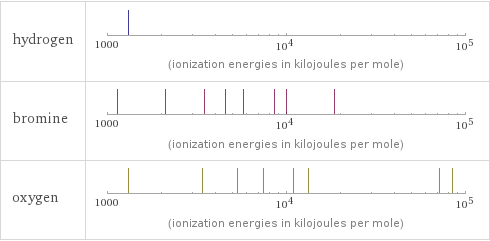
Reactivity
Atomic properties
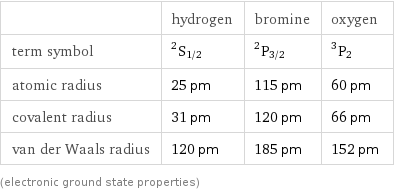
| hydrogen | bromine | oxygen term symbol | ^2S_(1/2) | ^2P_(3/2) | ^3P_2 atomic radius | 25 pm | 115 pm | 60 pm covalent radius | 31 pm | 120 pm | 66 pm van der Waals radius | 120 pm | 185 pm | 152 pm (electronic ground state properties)
Abundances
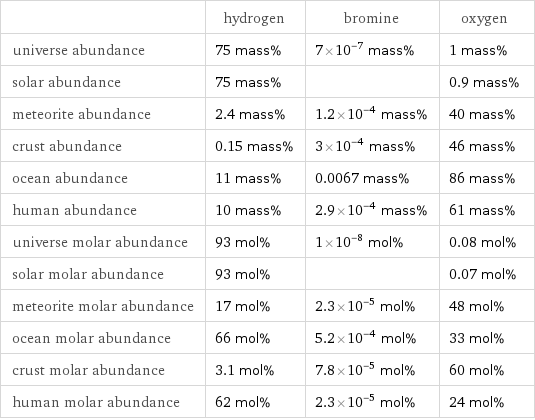
| hydrogen | bromine | oxygen universe abundance | 75 mass% | 7×10^-7 mass% | 1 mass% solar abundance | 75 mass% | | 0.9 mass% meteorite abundance | 2.4 mass% | 1.2×10^-4 mass% | 40 mass% crust abundance | 0.15 mass% | 3×10^-4 mass% | 46 mass% ocean abundance | 11 mass% | 0.0067 mass% | 86 mass% human abundance | 10 mass% | 2.9×10^-4 mass% | 61 mass% universe molar abundance | 93 mol% | 1×10^-8 mol% | 0.08 mol% solar molar abundance | 93 mol% | | 0.07 mol% meteorite molar abundance | 17 mol% | 2.3×10^-5 mol% | 48 mol% ocean molar abundance | 66 mol% | 5.2×10^-4 mol% | 33 mol% crust molar abundance | 3.1 mol% | 7.8×10^-5 mol% | 60 mol% human molar abundance | 62 mol% | 2.3×10^-5 mol% | 24 mol%