Input interpretation

scandium(III) sulfate pentahydrate | fluoboric acid
Chemical names and formulas
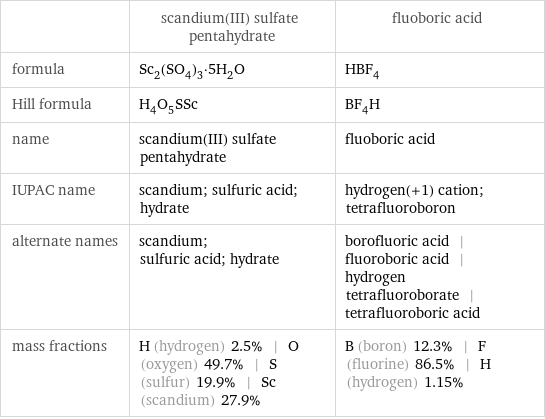
| scandium(III) sulfate pentahydrate | fluoboric acid formula | Sc_2(SO_4)_3·5H_2O | HBF_4 Hill formula | H_4O_5SSc | BF_4H name | scandium(III) sulfate pentahydrate | fluoboric acid IUPAC name | scandium; sulfuric acid; hydrate | hydrogen(+1) cation; tetrafluoroboron alternate names | scandium; sulfuric acid; hydrate | borofluoric acid | fluoroboric acid | hydrogen tetrafluoroborate | tetrafluoroboric acid mass fractions | H (hydrogen) 2.5% | O (oxygen) 49.7% | S (sulfur) 19.9% | Sc (scandium) 27.9% | B (boron) 12.3% | F (fluorine) 86.5% | H (hydrogen) 1.15%
Structure diagrams
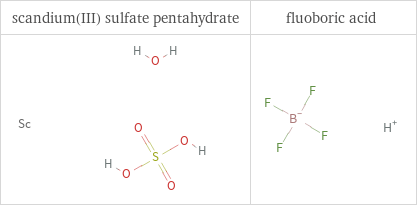
Structure diagrams
Basic properties
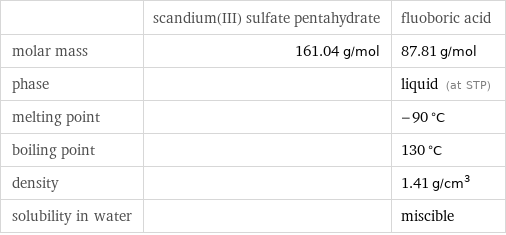
| scandium(III) sulfate pentahydrate | fluoboric acid molar mass | 161.04 g/mol | 87.81 g/mol phase | | liquid (at STP) melting point | | -90 °C boiling point | | 130 °C density | | 1.41 g/cm^3 solubility in water | | miscible
Units

Liquid properties

| fluoboric acid density | 1.41 g/cm^3 vapor pressure | 5 mmHg
Units
