Input interpretation
rubidium hydroxide
Chemical names and formulas
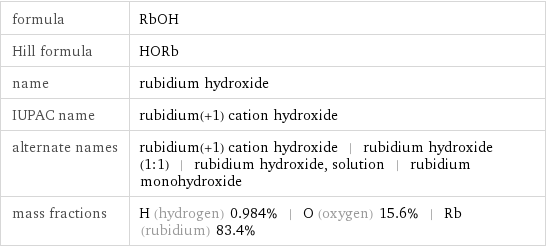
formula | RbOH Hill formula | HORb name | rubidium hydroxide IUPAC name | rubidium(+1) cation hydroxide alternate names | rubidium(+1) cation hydroxide | rubidium hydroxide (1:1) | rubidium hydroxide, solution | rubidium monohydroxide mass fractions | H (hydrogen) 0.984% | O (oxygen) 15.6% | Rb (rubidium) 83.4%
Structure diagram
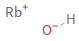
Structure diagram
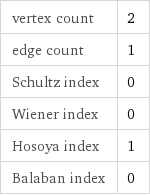
vertex count | 2 edge count | 1 Schultz index | 0 Wiener index | 0 Hosoya index | 1 Balaban index | 0
Basic properties
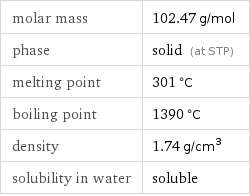
molar mass | 102.47 g/mol phase | solid (at STP) melting point | 301 °C boiling point | 1390 °C density | 1.74 g/cm^3 solubility in water | soluble
Units

Solid properties (at STP)
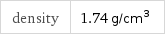
density | 1.74 g/cm^3
Units

Thermodynamic properties
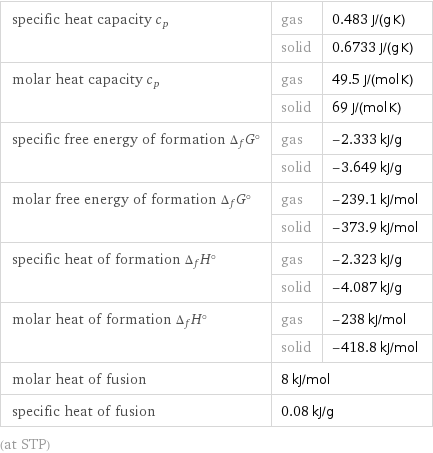
specific heat capacity c_p | gas | 0.483 J/(g K) | solid | 0.6733 J/(g K) molar heat capacity c_p | gas | 49.5 J/(mol K) | solid | 69 J/(mol K) specific free energy of formation Δ_fG° | gas | -2.333 kJ/g | solid | -3.649 kJ/g molar free energy of formation Δ_fG° | gas | -239.1 kJ/mol | solid | -373.9 kJ/mol specific heat of formation Δ_fH° | gas | -2.323 kJ/g | solid | -4.087 kJ/g molar heat of formation Δ_fH° | gas | -238 kJ/mol | solid | -418.8 kJ/mol molar heat of fusion | 8 kJ/mol | specific heat of fusion | 0.08 kJ/g | (at STP)
Chemical identifiers
![CAS number | 1310-82-3 PubChem CID number | 62393 PubChem SID number | 24854632 SMILES identifier | [OH-].[Rb+] InChI identifier | InChI=1/H2O.Rb/h1H2;/q;+1/p-1/fHO.Rb/h1h;/q-1;m RTECS number | VL8750000 MDL number | MFCD00011194](../image_source/466ca8aa8b55efa7bd6d955457718087.png)
CAS number | 1310-82-3 PubChem CID number | 62393 PubChem SID number | 24854632 SMILES identifier | [OH-].[Rb+] InChI identifier | InChI=1/H2O.Rb/h1H2;/q;+1/p-1/fHO.Rb/h1h;/q-1;m RTECS number | VL8750000 MDL number | MFCD00011194
NFPA label

NFPA label

NFPA health rating | 3 NFPA fire rating | 0 NFPA reactivity rating | 0
Toxicity properties

RTECS classes | other
Ion equivalents

Rb^+ (rubidium cation) | 1 (OH)^- (hydroxide anion) | 1