Input interpretation

heteronuclear diatomic molecules | molar heat of fusion
Summary
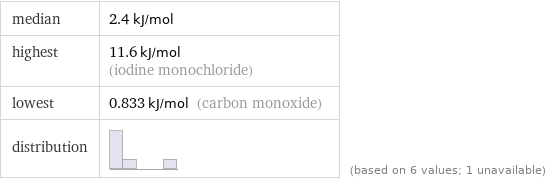
median | 2.4 kJ/mol highest | 11.6 kJ/mol (iodine monochloride) lowest | 0.833 kJ/mol (carbon monoxide) distribution | | (based on 6 values; 1 unavailable)
Entity with missing value
iodine bromide
Ranked values
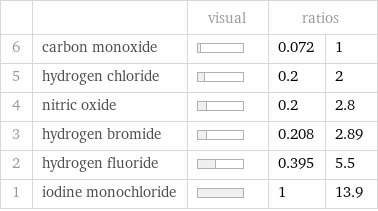
| | visual | ratios | 6 | carbon monoxide | | 0.072 | 1 5 | hydrogen chloride | | 0.2 | 2 4 | nitric oxide | | 0.2 | 2.8 3 | hydrogen bromide | | 0.208 | 2.89 2 | hydrogen fluoride | | 0.395 | 5.5 1 | iodine monochloride | | 1 | 13.9
Molar heat of fusion rankings
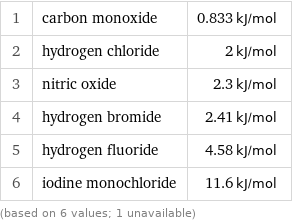
1 | carbon monoxide | 0.833 kJ/mol 2 | hydrogen chloride | 2 kJ/mol 3 | nitric oxide | 2.3 kJ/mol 4 | hydrogen bromide | 2.41 kJ/mol 5 | hydrogen fluoride | 4.58 kJ/mol 6 | iodine monochloride | 11.6 kJ/mol (based on 6 values; 1 unavailable)
Unit conversions for median molar heat of fusion 2.4 kJ/mol
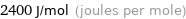
2400 J/mol (joules per mole)
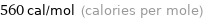
560 cal/mol (calories per mole)

0.56 kcal/mol (thermochemical kilocalories per mole)

0.024 eV (molar electronvolts)