Input interpretation

lithium perchlorate | ferric nitrate
Chemical names and formulas
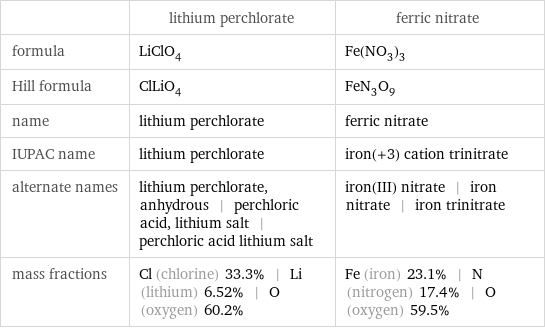
| lithium perchlorate | ferric nitrate formula | LiClO_4 | Fe(NO_3)_3 Hill formula | ClLiO_4 | FeN_3O_9 name | lithium perchlorate | ferric nitrate IUPAC name | lithium perchlorate | iron(+3) cation trinitrate alternate names | lithium perchlorate, anhydrous | perchloric acid, lithium salt | perchloric acid lithium salt | iron(III) nitrate | iron nitrate | iron trinitrate mass fractions | Cl (chlorine) 33.3% | Li (lithium) 6.52% | O (oxygen) 60.2% | Fe (iron) 23.1% | N (nitrogen) 17.4% | O (oxygen) 59.5%
Structure diagrams
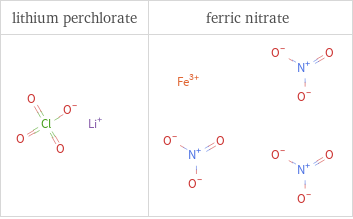
Structure diagrams
Basic properties
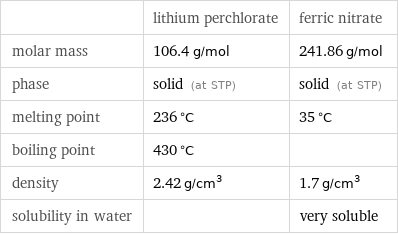
| lithium perchlorate | ferric nitrate molar mass | 106.4 g/mol | 241.86 g/mol phase | solid (at STP) | solid (at STP) melting point | 236 °C | 35 °C boiling point | 430 °C | density | 2.42 g/cm^3 | 1.7 g/cm^3 solubility in water | | very soluble
Units
