Input interpretation

nitrogen | reduced mass
Result
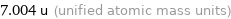
7.004 u (unified atomic mass units)
Unit conversions
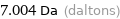
7.004 Da (daltons)
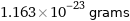
1.163×10^-23 grams
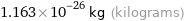
1.163×10^-26 kg (kilograms)
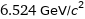
6.524 GeV/c^2
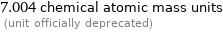
7.004 chemical atomic mass units (unit officially deprecated)
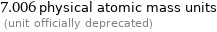
7.006 physical atomic mass units (unit officially deprecated)
Comparisons as mass
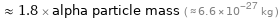
≈ 1.8 × alpha particle mass ( ≈ 6.6×10^-27 kg )
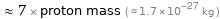
≈ 7 × proton mass ( ≈ 1.7×10^-27 kg )
Corresponding quantities
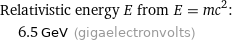
Relativistic energy E from E = mc^2: | 6.5 GeV (gigaelectronvolts)
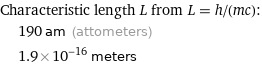
Characteristic length L from L = h/(mc): | 190 am (attometers) | 1.9×10^-16 meters
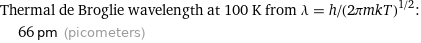
Thermal de Broglie wavelength at 100 K from λ = h/(2πmkT)^(1/2): | 66 pm (picometers)
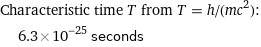
Characteristic time T from T = h/(mc^2): | 6.3×10^-25 seconds
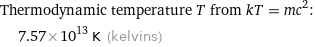
Thermodynamic temperature T from kT = mc^2: | 7.57×10^13 K (kelvins)
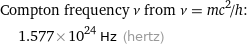
Compton frequency ν from ν = mc^2/h: | 1.577×10^24 Hz (hertz)