Input interpretation
magnesium oxide | potassium manganate
Chemical names and formulas
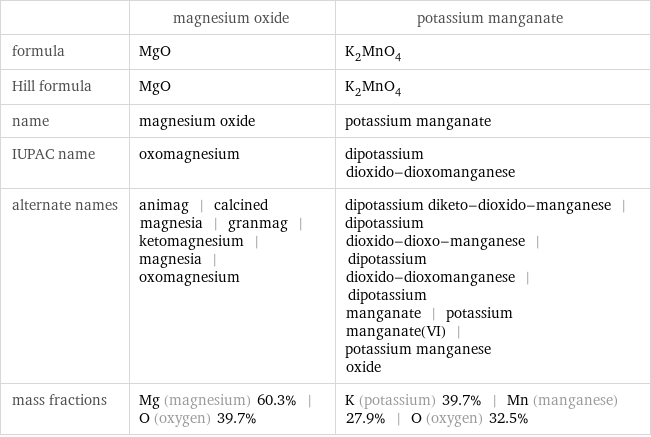
| magnesium oxide | potassium manganate formula | MgO | K_2MnO_4 Hill formula | MgO | K_2MnO_4 name | magnesium oxide | potassium manganate IUPAC name | oxomagnesium | dipotassium dioxido-dioxomanganese alternate names | animag | calcined magnesia | granmag | ketomagnesium | magnesia | oxomagnesium | dipotassium diketo-dioxido-manganese | dipotassium dioxido-dioxo-manganese | dipotassium dioxido-dioxomanganese | dipotassium manganate | potassium manganate(VI) | potassium manganese oxide mass fractions | Mg (magnesium) 60.3% | O (oxygen) 39.7% | K (potassium) 39.7% | Mn (manganese) 27.9% | O (oxygen) 32.5%
Structure diagrams
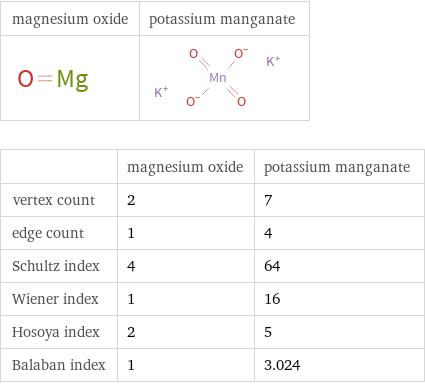
| magnesium oxide | potassium manganate vertex count | 2 | 7 edge count | 1 | 4 Schultz index | 4 | 64 Wiener index | 1 | 16 Hosoya index | 2 | 5 Balaban index | 1 | 3.024
Basic properties
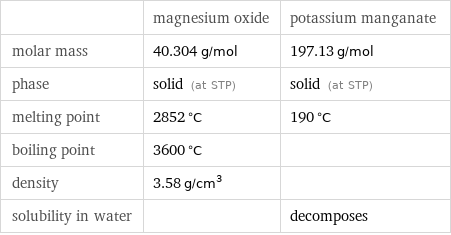
| magnesium oxide | potassium manganate molar mass | 40.304 g/mol | 197.13 g/mol phase | solid (at STP) | solid (at STP) melting point | 2852 °C | 190 °C boiling point | 3600 °C | density | 3.58 g/cm^3 | solubility in water | | decomposes
Units

Thermodynamic properties
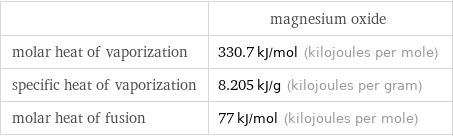
| magnesium oxide molar heat of vaporization | 330.7 kJ/mol (kilojoules per mole) specific heat of vaporization | 8.205 kJ/g (kilojoules per gram) molar heat of fusion | 77 kJ/mol (kilojoules per mole)
Solid properties (at STP)

| magnesium oxide density | 3.58 g/cm^3 vapor pressure | 0.3 mmHg
Units

Chemical identifiers
![| magnesium oxide | potassium manganate CAS number | 1309-48-4 | 10294-64-1 PubChem CID number | 14792 | 160931 PubChem SID number | 24852193 | 24853025 SMILES identifier | O=[Mg] | [O-][Mn](=O)(=O)[O-].[K+].[K+]](../image_source/27c185746054e9d81d067c59fb73772b.png)
| magnesium oxide | potassium manganate CAS number | 1309-48-4 | 10294-64-1 PubChem CID number | 14792 | 160931 PubChem SID number | 24852193 | 24853025 SMILES identifier | O=[Mg] | [O-][Mn](=O)(=O)[O-].[K+].[K+]