Input interpretation

metallic elements | atomic volume
Summary
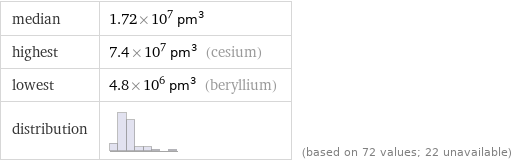
median | 1.72×10^7 pm^3 highest | 7.4×10^7 pm^3 (cesium) lowest | 4.8×10^6 pm^3 (beryllium) distribution | | (based on 72 values; 22 unavailable)
Entities with missing values
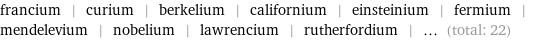
francium | curium | berkelium | californium | einsteinium | fermium | mendelevium | nobelium | lawrencium | rutherfordium | ... (total: 22)
Plot
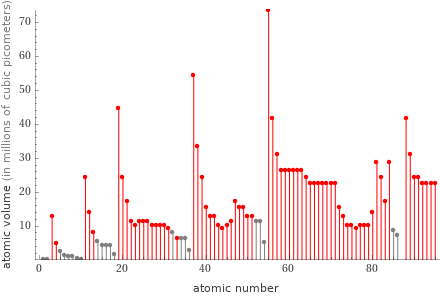
Plot
Periodic table location
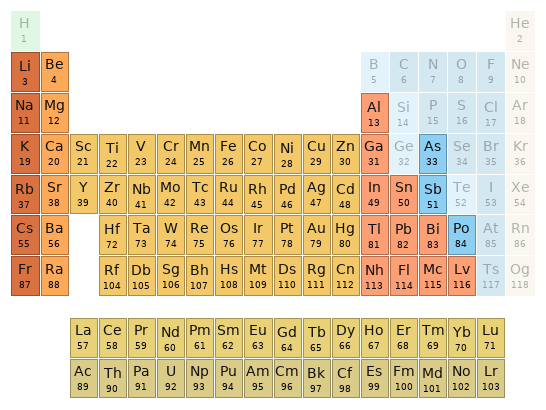
Periodic table location
Atomic radii properties
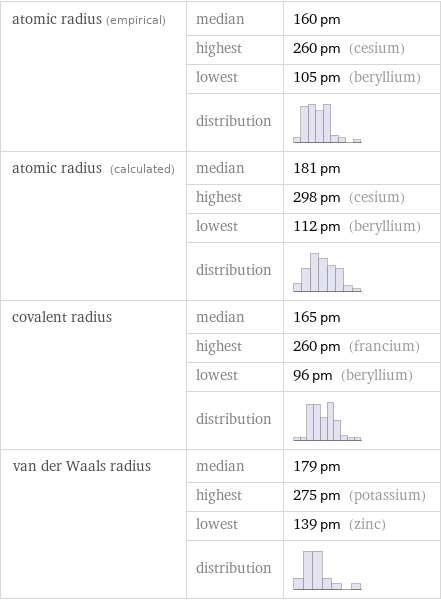
atomic radius (empirical) | median | 160 pm | highest | 260 pm (cesium) | lowest | 105 pm (beryllium) | distribution | atomic radius (calculated) | median | 181 pm | highest | 298 pm (cesium) | lowest | 112 pm (beryllium) | distribution | covalent radius | median | 165 pm | highest | 260 pm (francium) | lowest | 96 pm (beryllium) | distribution | van der Waals radius | median | 179 pm | highest | 275 pm (potassium) | lowest | 139 pm (zinc) | distribution |
Units

Distribution plots
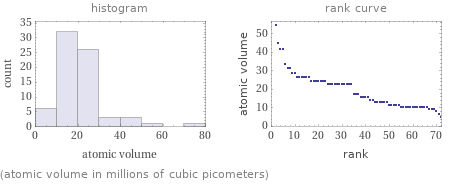
(atomic volume in millions of cubic picometers)
Atomic volume rankings
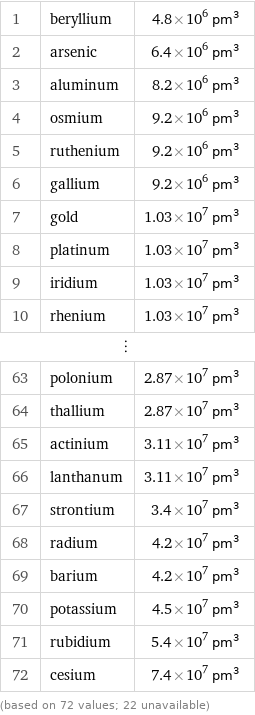
1 | beryllium | 4.8×10^6 pm^3 2 | arsenic | 6.4×10^6 pm^3 3 | aluminum | 8.2×10^6 pm^3 4 | osmium | 9.2×10^6 pm^3 5 | ruthenium | 9.2×10^6 pm^3 6 | gallium | 9.2×10^6 pm^3 7 | gold | 1.03×10^7 pm^3 8 | platinum | 1.03×10^7 pm^3 9 | iridium | 1.03×10^7 pm^3 10 | rhenium | 1.03×10^7 pm^3 ⋮ | | 63 | polonium | 2.87×10^7 pm^3 64 | thallium | 2.87×10^7 pm^3 65 | actinium | 3.11×10^7 pm^3 66 | lanthanum | 3.11×10^7 pm^3 67 | strontium | 3.4×10^7 pm^3 68 | radium | 4.2×10^7 pm^3 69 | barium | 4.2×10^7 pm^3 70 | potassium | 4.5×10^7 pm^3 71 | rubidium | 5.4×10^7 pm^3 72 | cesium | 7.4×10^7 pm^3 (based on 72 values; 22 unavailable)
Unit conversions for median atomic volume 1.72×10^7 pm^3
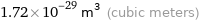
1.72×10^-29 m^3 (cubic meters)
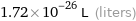
1.72×10^-26 L (liters)
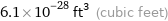
6.1×10^-28 ft^3 (cubic feet)
Corresponding quantities

Radius r of a sphere from V = 4πr^3/3: | 160 pm (picometers) | 6.3×10^-9 inches

Edge length a of a cube from V = a^3: | 258 pm (picometers) | 1×10^-8 inches