Input interpretation
boron | europium(III) nitrate hydrate
Chemical names and formulas
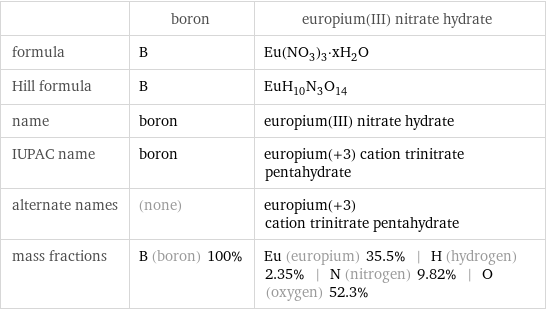
| boron | europium(III) nitrate hydrate formula | B | Eu(NO_3)_3·xH_2O Hill formula | B | EuH_10N_3O_14 name | boron | europium(III) nitrate hydrate IUPAC name | boron | europium(+3) cation trinitrate pentahydrate alternate names | (none) | europium(+3) cation trinitrate pentahydrate mass fractions | B (boron) 100% | Eu (europium) 35.5% | H (hydrogen) 2.35% | N (nitrogen) 9.82% | O (oxygen) 52.3%
Structure diagrams
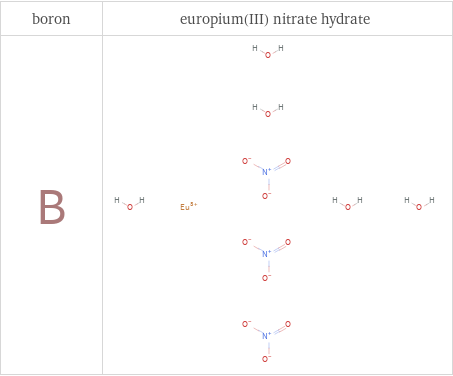
Structure diagrams
Basic properties
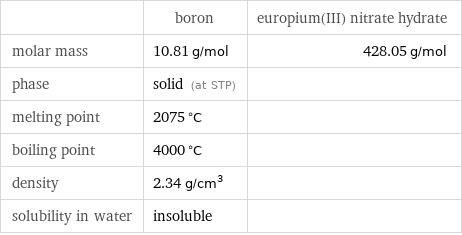
| boron | europium(III) nitrate hydrate molar mass | 10.81 g/mol | 428.05 g/mol phase | solid (at STP) | melting point | 2075 °C | boiling point | 4000 °C | density | 2.34 g/cm^3 | solubility in water | insoluble |
Units

Thermodynamic properties
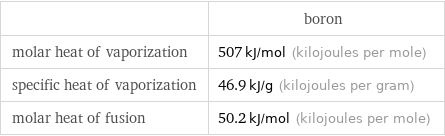
| boron molar heat of vaporization | 507 kJ/mol (kilojoules per mole) specific heat of vaporization | 46.9 kJ/g (kilojoules per gram) molar heat of fusion | 50.2 kJ/mol (kilojoules per mole)
Solid properties
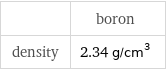
| boron density | 2.34 g/cm^3
Units
