Input interpretation

Na_2S sodium sulfide + AgF silver fluoride ⟶ Ag_2S silver(I) sulfide + NaF sodium fluoride
Chemical names and formulas
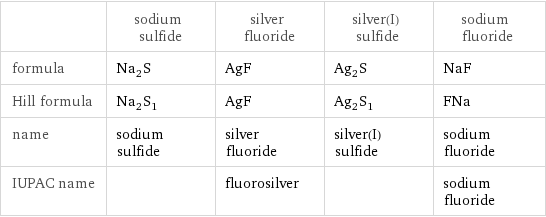
| sodium sulfide | silver fluoride | silver(I) sulfide | sodium fluoride formula | Na_2S | AgF | Ag_2S | NaF Hill formula | Na_2S_1 | AgF | Ag_2S_1 | FNa name | sodium sulfide | silver fluoride | silver(I) sulfide | sodium fluoride IUPAC name | | fluorosilver | | sodium fluoride
Substance properties
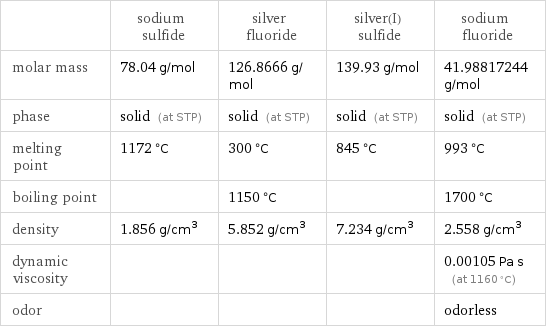
| sodium sulfide | silver fluoride | silver(I) sulfide | sodium fluoride molar mass | 78.04 g/mol | 126.8666 g/mol | 139.93 g/mol | 41.98817244 g/mol phase | solid (at STP) | solid (at STP) | solid (at STP) | solid (at STP) melting point | 1172 °C | 300 °C | 845 °C | 993 °C boiling point | | 1150 °C | | 1700 °C density | 1.856 g/cm^3 | 5.852 g/cm^3 | 7.234 g/cm^3 | 2.558 g/cm^3 dynamic viscosity | | | | 0.00105 Pa s (at 1160 °C) odor | | | | odorless
Units
