Input interpretation

gaseous elements (at STP) | melting point
Summary
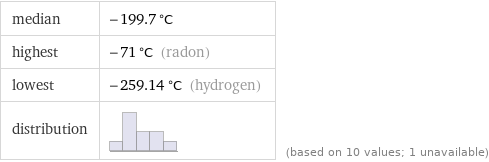
median | -199.7 °C highest | -71 °C (radon) lowest | -259.14 °C (hydrogen) distribution | | (based on 10 values; 1 unavailable)
Entity with missing value
helium
Plot
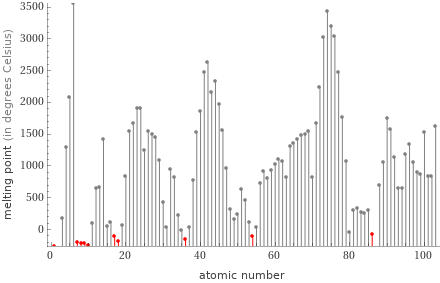
Plot
Distribution plots
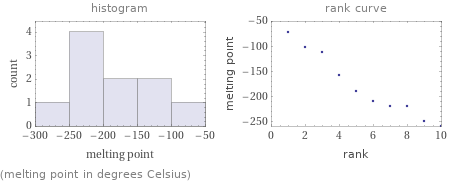
(melting point in degrees Celsius)
Melting point rankings
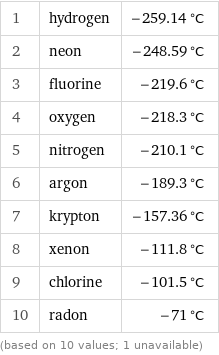
1 | hydrogen | -259.14 °C 2 | neon | -248.59 °C 3 | fluorine | -219.6 °C 4 | oxygen | -218.3 °C 5 | nitrogen | -210.1 °C 6 | argon | -189.3 °C 7 | krypton | -157.36 °C 8 | xenon | -111.8 °C 9 | chlorine | -101.5 °C 10 | radon | -71 °C (based on 10 values; 1 unavailable)
Unit conversions for median melting point -199.7 °C
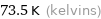
73.5 K (kelvins)

-327.5 °F (degrees Fahrenheit)
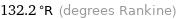
132.2 °R (degrees Rankine)
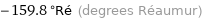
-159.8 °Ré (degrees Réaumur)

-97.3 °Rø (degrees Rømer)
Comparison for median melting point -199.7 °C

3.9 °C below boiling point of nitrogen (-195.79 °C)
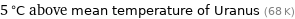
5 °C above mean temperature of Uranus (68 K)
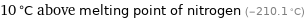
10 °C above melting point of nitrogen (-210.1 °C)
Corresponding quantities

Thermodynamic energy E from E = kT: | 6.3 meV (millielectronvolts)
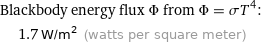
Blackbody energy flux Φ from Φ = σT^4: | 1.7 W/m^2 (watts per square meter)
Thermodynamic properties
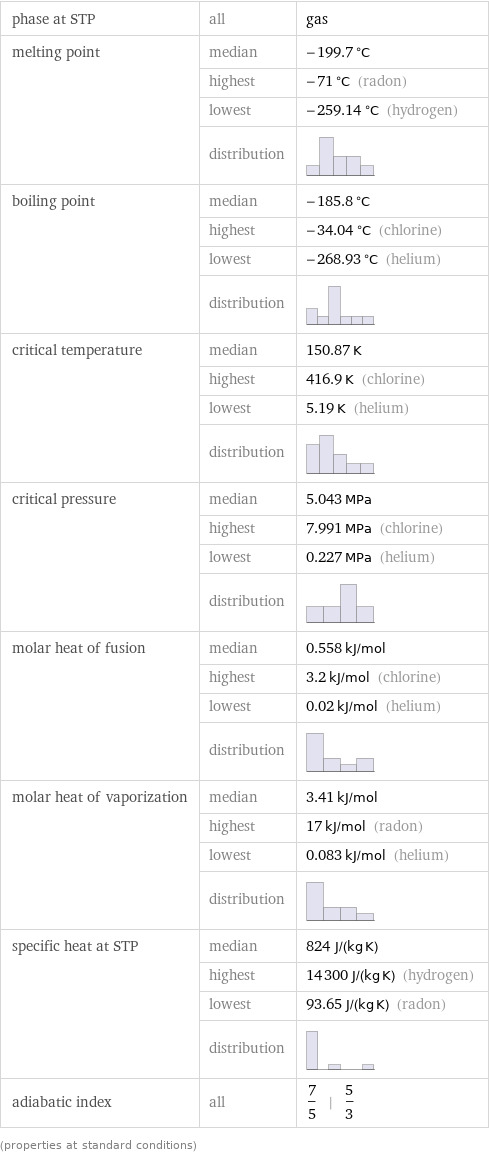
phase at STP | all | gas melting point | median | -199.7 °C | highest | -71 °C (radon) | lowest | -259.14 °C (hydrogen) | distribution | boiling point | median | -185.8 °C | highest | -34.04 °C (chlorine) | lowest | -268.93 °C (helium) | distribution | critical temperature | median | 150.87 K | highest | 416.9 K (chlorine) | lowest | 5.19 K (helium) | distribution | critical pressure | median | 5.043 MPa | highest | 7.991 MPa (chlorine) | lowest | 0.227 MPa (helium) | distribution | molar heat of fusion | median | 0.558 kJ/mol | highest | 3.2 kJ/mol (chlorine) | lowest | 0.02 kJ/mol (helium) | distribution | molar heat of vaporization | median | 3.41 kJ/mol | highest | 17 kJ/mol (radon) | lowest | 0.083 kJ/mol (helium) | distribution | specific heat at STP | median | 824 J/(kg K) | highest | 14300 J/(kg K) (hydrogen) | lowest | 93.65 J/(kg K) (radon) | distribution | adiabatic index | all | 7/5 | 5/3 (properties at standard conditions)