Input interpretation
nocardicin c
Chemical names and formulas

formula | C_23H_26N_4O_8 name | nocardicin c mass fractions | C (carbon) 57.5% | H (hydrogen) 4.2% | N (nitrogen) 11.7% | O (oxygen) 26.6%
Lewis structure
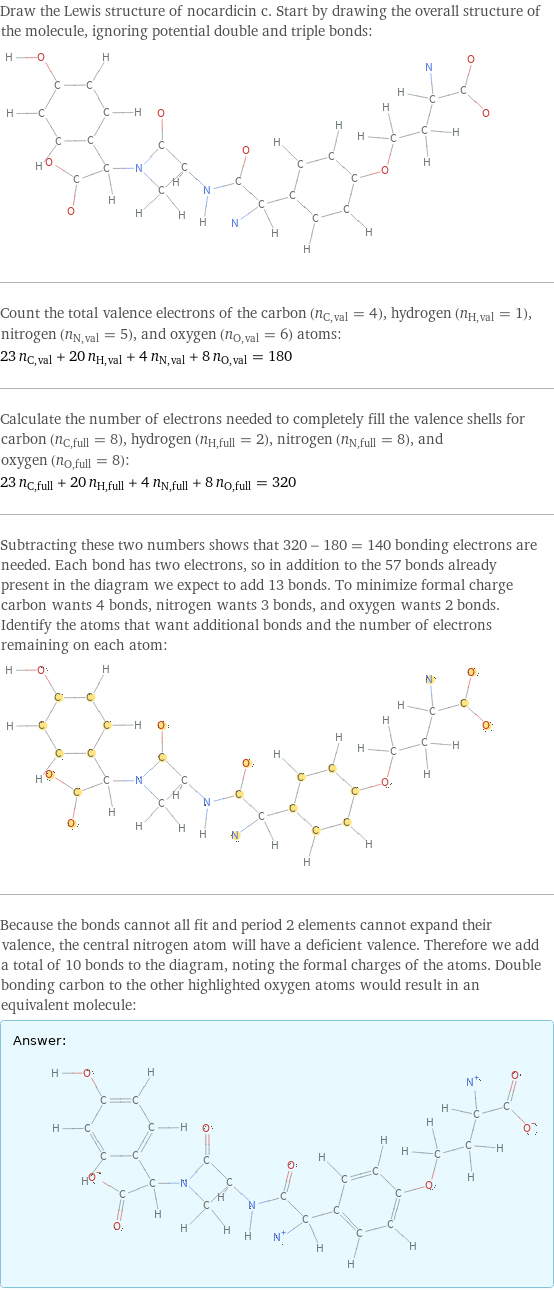
Draw the Lewis structure of nocardicin c. Start by drawing the overall structure of the molecule, ignoring potential double and triple bonds: Count the total valence electrons of the carbon (n_C, val = 4), hydrogen (n_H, val = 1), nitrogen (n_N, val = 5), and oxygen (n_O, val = 6) atoms: 23 n_C, val + 20 n_H, val + 4 n_N, val + 8 n_O, val = 180 Calculate the number of electrons needed to completely fill the valence shells for carbon (n_C, full = 8), hydrogen (n_H, full = 2), nitrogen (n_N, full = 8), and oxygen (n_O, full = 8): 23 n_C, full + 20 n_H, full + 4 n_N, full + 8 n_O, full = 320 Subtracting these two numbers shows that 320 - 180 = 140 bonding electrons are needed. Each bond has two electrons, so in addition to the 57 bonds already present in the diagram we expect to add 13 bonds. To minimize formal charge carbon wants 4 bonds, nitrogen wants 3 bonds, and oxygen wants 2 bonds. Identify the atoms that want additional bonds and the number of electrons remaining on each atom: Because the bonds cannot all fit and period 2 elements cannot expand their valence, the central nitrogen atom will have a deficient valence. Therefore we add a total of 10 bonds to the diagram, noting the formal charges of the atoms. Double bonding carbon to the other highlighted oxygen atoms would result in an equivalent molecule: Answer: | |
Basic properties

molar mass | 480.43 g/mol
Units

Chemical identifiers
![PubChem CID number | 90657582 SMILES identifier | C3(C(NC(C(C1(=CC=C(OCCC([N+])C(=O)[O-])C=C1))[N+])=O)C(=O)N(C(C2(=CC=C(O)C=C2))C(=O)[O-])3)](../image_source/10a22f479dffa32d312077378b2d8885.png)
PubChem CID number | 90657582 SMILES identifier | C3(C(NC(C(C1(=CC=C(OCCC([N+])C(=O)[O-])C=C1))[N+])=O)C(=O)N(C(C2(=CC=C(O)C=C2))C(=O)[O-])3)