Input interpretation

vanadium(V) oxychloride | fluoboric acid
Chemical names and formulas
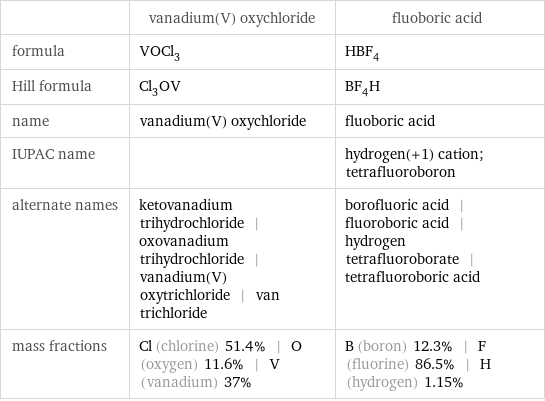
| vanadium(V) oxychloride | fluoboric acid formula | VOCl_3 | HBF_4 Hill formula | Cl_3OV | BF_4H name | vanadium(V) oxychloride | fluoboric acid IUPAC name | | hydrogen(+1) cation; tetrafluoroboron alternate names | ketovanadium trihydrochloride | oxovanadium trihydrochloride | vanadium(V) oxytrichloride | van trichloride | borofluoric acid | fluoroboric acid | hydrogen tetrafluoroborate | tetrafluoroboric acid mass fractions | Cl (chlorine) 51.4% | O (oxygen) 11.6% | V (vanadium) 37% | B (boron) 12.3% | F (fluorine) 86.5% | H (hydrogen) 1.15%
Structure diagrams
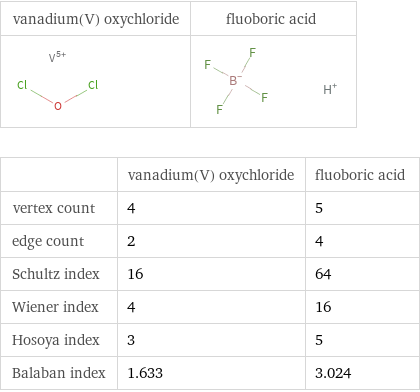
| vanadium(V) oxychloride | fluoboric acid vertex count | 4 | 5 edge count | 2 | 4 Schultz index | 16 | 64 Wiener index | 4 | 16 Hosoya index | 3 | 5 Balaban index | 1.633 | 3.024
Basic properties
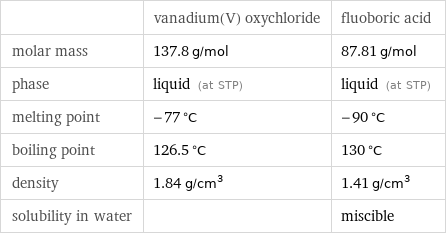
| vanadium(V) oxychloride | fluoboric acid molar mass | 137.8 g/mol | 87.81 g/mol phase | liquid (at STP) | liquid (at STP) melting point | -77 °C | -90 °C boiling point | 126.5 °C | 130 °C density | 1.84 g/cm^3 | 1.41 g/cm^3 solubility in water | | miscible
Units

Liquid properties

| vanadium(V) oxychloride | fluoboric acid density | 1.84 g/cm^3 | 1.41 g/cm^3 vapor pressure | 13.8 mmHg | 5 mmHg
Units

Chemical identifiers
![| vanadium(V) oxychloride | fluoboric acid CAS number | 7727-18-6 | 16872-11-0 PubChem CID number | | 28118 SMILES identifier | ClOCl.[V+5] | [H+].[B-](F)(F)(F)F](../image_source/dca7fcfdb284e2040ac9423a39e561d3.png)
| vanadium(V) oxychloride | fluoboric acid CAS number | 7727-18-6 | 16872-11-0 PubChem CID number | | 28118 SMILES identifier | ClOCl.[V+5] | [H+].[B-](F)(F)(F)F