Input interpretation
sym-tetrachloroethane
Chemical names and formulas
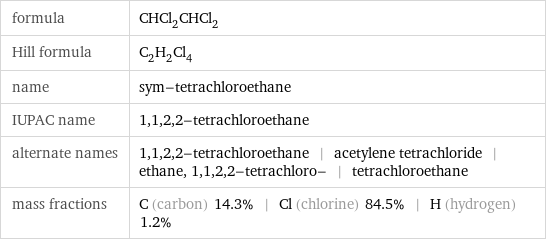
formula | CHCl_2CHCl_2 Hill formula | C_2H_2Cl_4 name | sym-tetrachloroethane IUPAC name | 1, 1, 2, 2-tetrachloroethane alternate names | 1, 1, 2, 2-tetrachloroethane | acetylene tetrachloride | ethane, 1, 1, 2, 2-tetrachloro- | tetrachloroethane mass fractions | C (carbon) 14.3% | Cl (chlorine) 84.5% | H (hydrogen) 1.2%
Lewis structure
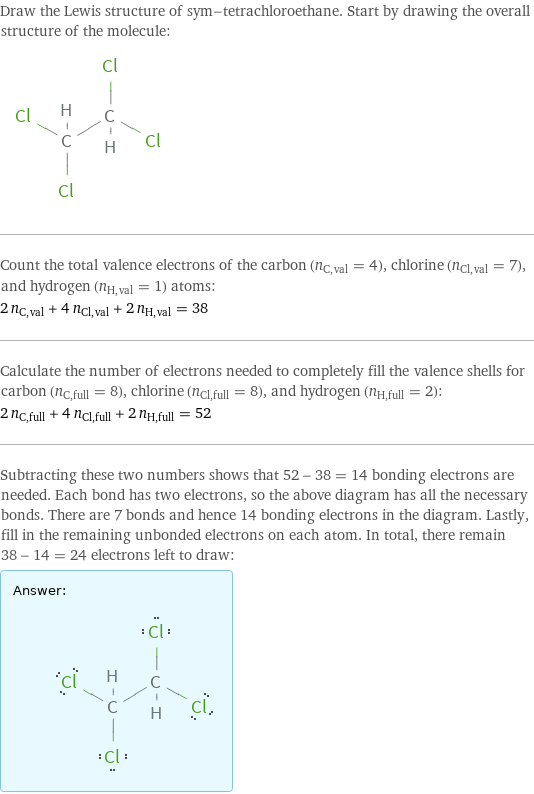
Draw the Lewis structure of sym-tetrachloroethane. Start by drawing the overall structure of the molecule: Count the total valence electrons of the carbon (n_C, val = 4), chlorine (n_Cl, val = 7), and hydrogen (n_H, val = 1) atoms: 2 n_C, val + 4 n_Cl, val + 2 n_H, val = 38 Calculate the number of electrons needed to completely fill the valence shells for carbon (n_C, full = 8), chlorine (n_Cl, full = 8), and hydrogen (n_H, full = 2): 2 n_C, full + 4 n_Cl, full + 2 n_H, full = 52 Subtracting these two numbers shows that 52 - 38 = 14 bonding electrons are needed. Each bond has two electrons, so the above diagram has all the necessary bonds. There are 7 bonds and hence 14 bonding electrons in the diagram. Lastly, fill in the remaining unbonded electrons on each atom. In total, there remain 38 - 14 = 24 electrons left to draw: Answer: | |
3D structure
3D structure
Basic properties
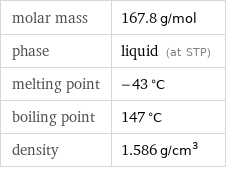
molar mass | 167.8 g/mol phase | liquid (at STP) melting point | -43 °C boiling point | 147 °C density | 1.586 g/cm^3
Units

Liquid properties (at STP)
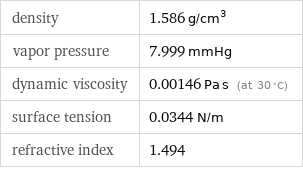
density | 1.586 g/cm^3 vapor pressure | 7.999 mmHg dynamic viscosity | 0.00146 Pa s (at 30 °C) surface tension | 0.0344 N/m refractive index | 1.494
Units
Thermodynamic properties
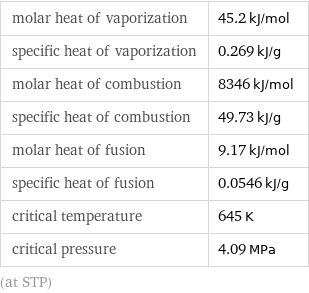
molar heat of vaporization | 45.2 kJ/mol specific heat of vaporization | 0.269 kJ/g molar heat of combustion | 8346 kJ/mol specific heat of combustion | 49.73 kJ/g molar heat of fusion | 9.17 kJ/mol specific heat of fusion | 0.0546 kJ/g critical temperature | 645 K critical pressure | 4.09 MPa (at STP)
Chemical identifiers
CAS number | 79-34-5 Beilstein number | 969206 PubChem CID number | 6591 SMILES identifier | C(C(Cl)Cl)(Cl)Cl InChI identifier | InChI=1/C2H2Cl4/c3-1(4)2(5)6/h1-2H RTECS number | KI8575000 MDL number | MFCD00000848
NFPA label

NFPA label

NFPA health rating | 3 NFPA fire rating | 0 NFPA reactivity rating | 0
Safety properties

flash point | 62 °C

DOT hazard class | 6.1 DOT numbers | 1702
Toxicity properties

threshold limit value | 1 ppmv

RTECS classes | agricultural chemical and pesticide | tumorigen | drug | mutagen | human data