Input interpretation
beryllium fluoride
Chemical names and formulas

formula | BeF_2 name | beryllium fluoride IUPAC name | beryllium(+2) cation difluoride alternate names | beryllium difluoride | fluoroberyllate mass fractions | Be (beryllium) 19.2% | F (fluorine) 80.8%
Structure diagram
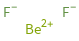
Structure diagram
Basic properties
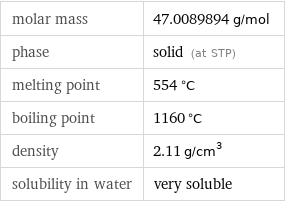
molar mass | 47.0089894 g/mol phase | solid (at STP) melting point | 554 °C boiling point | 1160 °C density | 2.11 g/cm^3 solubility in water | very soluble
Units

Solid properties (at STP)
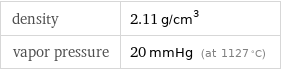
density | 2.11 g/cm^3 vapor pressure | 20 mmHg (at 1127 °C)
Units

Thermodynamic properties
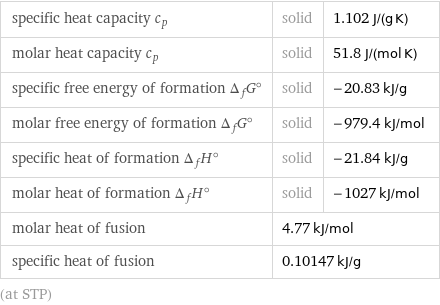
specific heat capacity c_p | solid | 1.102 J/(g K) molar heat capacity c_p | solid | 51.8 J/(mol K) specific free energy of formation Δ_fG° | solid | -20.83 kJ/g molar free energy of formation Δ_fG° | solid | -979.4 kJ/mol specific heat of formation Δ_fH° | solid | -21.84 kJ/g molar heat of formation Δ_fH° | solid | -1027 kJ/mol molar heat of fusion | 4.77 kJ/mol | specific heat of fusion | 0.10147 kJ/g | (at STP)
Chemical identifiers
![CAS number | 7787-49-7 PubChem CID number | 24589 SMILES identifier | [Be+2].[F-].[F-] InChI identifier | InChI=1/Be.2FH/h;2*1H/q+2;;/p-2/fBe.2F/h;2*1h/qm;2*-1 EU number | 232-118-5 Gmelin number | 95146 RTECS number | DS2800000 NSC number | 84264](../image_source/9154c10c7047d629ef4df24f332604ed.png)
CAS number | 7787-49-7 PubChem CID number | 24589 SMILES identifier | [Be+2].[F-].[F-] InChI identifier | InChI=1/Be.2FH/h;2*1H/q+2;;/p-2/fBe.2F/h;2*1h/qm;2*-1 EU number | 232-118-5 Gmelin number | 95146 RTECS number | DS2800000 NSC number | 84264
Toxicity properties

RTECS classes | tumorigen