Input interpretation

Lewis acids | molar heat of fusion
Summary

median | 9.2 kJ/mol highest | 40 kJ/mol (iron(III) chloride) lowest | 0.56 kJ/mol (aluminum fluoride) distribution | | (based on 7 values; 3 unavailable)
Entities with missing values
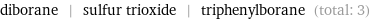
diborane | sulfur trioxide | triphenylborane (total: 3)
Molar heat of fusion rankings
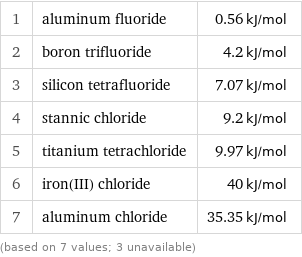
1 | aluminum fluoride | 0.56 kJ/mol 2 | boron trifluoride | 4.2 kJ/mol 3 | silicon tetrafluoride | 7.07 kJ/mol 4 | stannic chloride | 9.2 kJ/mol 5 | titanium tetrachloride | 9.97 kJ/mol 6 | iron(III) chloride | 40 kJ/mol 7 | aluminum chloride | 35.35 kJ/mol (based on 7 values; 3 unavailable)
Unit conversions for median molar heat of fusion 9.2 kJ/mol
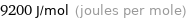
9200 J/mol (joules per mole)
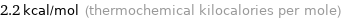
2.2 kcal/mol (thermochemical kilocalories per mole)
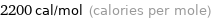
2200 cal/mol (calories per mole)

0.095 eV (molar electronvolts)