Input interpretation

oxidants | electron count
Summary
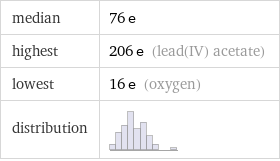
median | 76 e highest | 206 e (lead(IV) acetate) lowest | 16 e (oxygen) distribution |
Units

Distribution plots
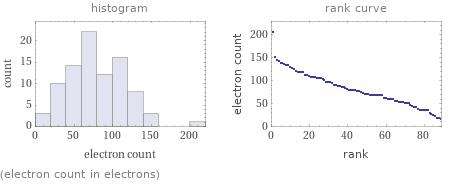
(electron count in electrons)
Electron count rankings
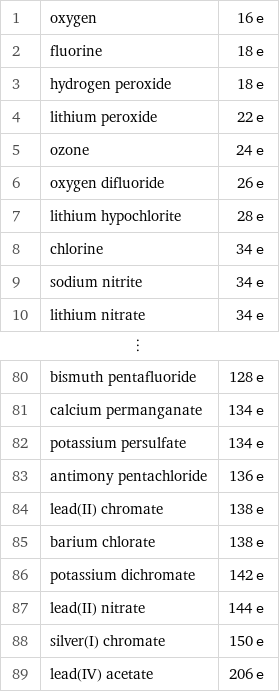
1 | oxygen | 16 e 2 | fluorine | 18 e 3 | hydrogen peroxide | 18 e 4 | lithium peroxide | 22 e 5 | ozone | 24 e 6 | oxygen difluoride | 26 e 7 | lithium hypochlorite | 28 e 8 | chlorine | 34 e 9 | sodium nitrite | 34 e 10 | lithium nitrate | 34 e ⋮ | | 80 | bismuth pentafluoride | 128 e 81 | calcium permanganate | 134 e 82 | potassium persulfate | 134 e 83 | antimony pentachloride | 136 e 84 | lead(II) chromate | 138 e 85 | barium chlorate | 138 e 86 | potassium dichromate | 142 e 87 | lead(II) nitrate | 144 e 88 | silver(I) chromate | 150 e 89 | lead(IV) acetate | 206 e