Input interpretation

weak electrolytes | electron count
Summary
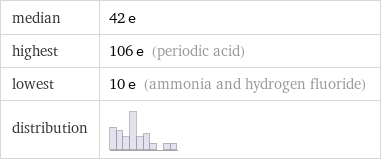
median | 42 e highest | 106 e (periodic acid) lowest | 10 e (ammonia and hydrogen fluoride) distribution |
Units

Distribution plots
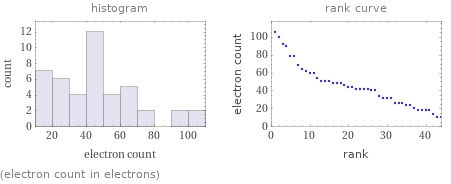
(electron count in electrons)
Electron count rankings
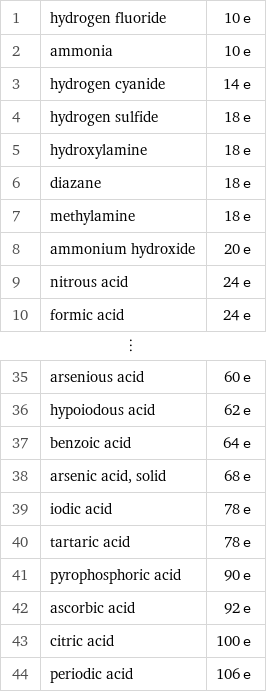
1 | hydrogen fluoride | 10 e 2 | ammonia | 10 e 3 | hydrogen cyanide | 14 e 4 | hydrogen sulfide | 18 e 5 | hydroxylamine | 18 e 6 | diazane | 18 e 7 | methylamine | 18 e 8 | ammonium hydroxide | 20 e 9 | nitrous acid | 24 e 10 | formic acid | 24 e ⋮ | | 35 | arsenious acid | 60 e 36 | hypoiodous acid | 62 e 37 | benzoic acid | 64 e 38 | arsenic acid, solid | 68 e 39 | iodic acid | 78 e 40 | tartaric acid | 78 e 41 | pyrophosphoric acid | 90 e 42 | ascorbic acid | 92 e 43 | citric acid | 100 e 44 | periodic acid | 106 e
Corresponding quantity
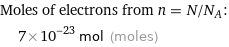
Moles of electrons from n = N/N_A: | 7×10^-23 mol (moles)