Input interpretation

sodium | work function
Results
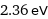
2.36 eV
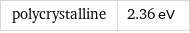
polycrystalline | 2.36 eV
Threshold frequency
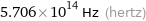
5.706×10^14 Hz (hertz)
Electromagnetic frequency range
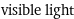
visible light
Unit conversion
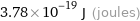
3.78×10^-19 J (joules)
Comparisons as energy
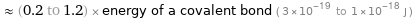
≈ (0.2 to 1.2) × energy of a covalent bond ( 3×10^-19 to 1×10^-18 J )

≈ (1 to 1) × energy of a photon of green light ( 2.2 to 2.5 eV )
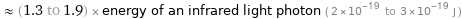
≈ (1.3 to 1.9) × energy of an infrared light photon ( 2×10^-19 to 3×10^-19 J )
Corresponding quantities

Electromagnetic wave frequency ν from E = hν: | 571 THz (terahertz) | 5.706×10^14 Hz (hertz)

Thermodynamic temperature T from E = kT: | 27387 K (kelvins)
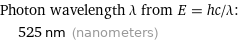
Photon wavelength λ from E = hc/λ: | 525 nm (nanometers)
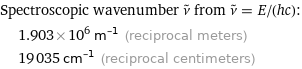
Spectroscopic wavenumber ν^~ from ν^~ = E/(hc): | 1.903×10^6 m^(-1) (reciprocal meters) | 19035 cm^(-1) (reciprocal centimeters)