Input interpretation

greenhouse gases | vapor pressure
Summary
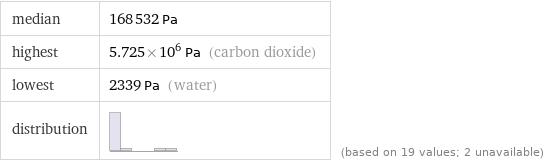
median | 168532 Pa highest | 5.725×10^6 Pa (carbon dioxide) lowest | 2339 Pa (water) distribution | | (based on 19 values; 2 unavailable)
Entities with missing values
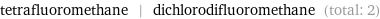
tetrafluoromethane | dichlorodifluoromethane (total: 2)
Distribution plots
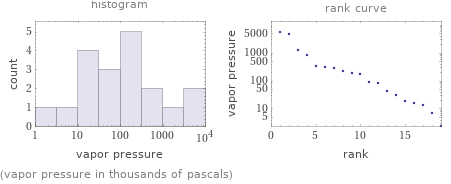
(vapor pressure in thousands of pascals)
Vapor pressure rankings
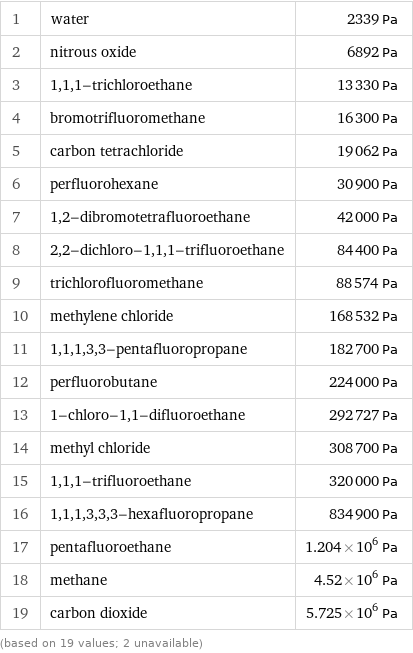
1 | water | 2339 Pa 2 | nitrous oxide | 6892 Pa 3 | 1, 1, 1-trichloroethane | 13330 Pa 4 | bromotrifluoromethane | 16300 Pa 5 | carbon tetrachloride | 19062 Pa 6 | perfluorohexane | 30900 Pa 7 | 1, 2-dibromotetrafluoroethane | 42000 Pa 8 | 2, 2-dichloro-1, 1, 1-trifluoroethane | 84400 Pa 9 | trichlorofluoromethane | 88574 Pa 10 | methylene chloride | 168532 Pa 11 | 1, 1, 1, 3, 3-pentafluoropropane | 182700 Pa 12 | perfluorobutane | 224000 Pa 13 | 1-chloro-1, 1-difluoroethane | 292727 Pa 14 | methyl chloride | 308700 Pa 15 | 1, 1, 1-trifluoroethane | 320000 Pa 16 | 1, 1, 1, 3, 3, 3-hexafluoropropane | 834900 Pa 17 | pentafluoroethane | 1.204×10^6 Pa 18 | methane | 4.52×10^6 Pa 19 | carbon dioxide | 5.725×10^6 Pa (based on 19 values; 2 unavailable)
Unit conversions for median vapor pressure 168532 Pa
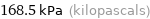
168.5 kPa (kilopascals)
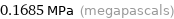
0.1685 MPa (megapascals)

168532 N/m^2 (newtons per square meter)
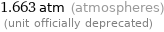
1.663 atm (atmospheres) (unit officially deprecated)

1.719 at (technical atmospheres) (unit officially deprecated)
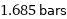
1.685 bars
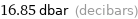
16.85 dbar (decibars)
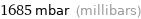
1685 mbar (millibars)
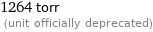
1264 torr (unit officially deprecated)
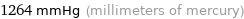
1264 mmHg (millimeters of mercury)
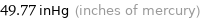
49.77 inHg (inches of mercury)
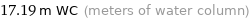
17.19 m WC (meters of water column)

0.01719 km WC (kilometers of water column)

676.6 in WC (inches of water column (conventional))
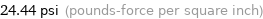
24.44 psi (pounds-force per square inch)
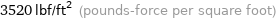
3520 lbf/ft^2 (pounds-force per square foot)
Comparisons for median vapor pressure 168532 Pa

≈ (0.4 to 0.8) × pressure inside a typical soda can at room temperature ( 207 to 380 kPa )
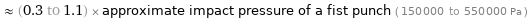
≈ (0.3 to 1.1) × approximate impact pressure of a fist punch ( 150000 to 550000 Pa )

≈ 0.83 × pressure in a typical, disposable cigarette lighter at 70 degrees Fahrenheit ( ≈ 2 atm )
Corresponding quantity
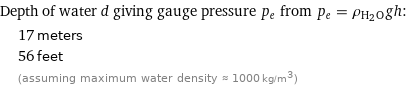
Depth of water d giving gauge pressure p_e from p_e = ρ_(H_2O)gh: | 17 meters | 56 feet | (assuming maximum water density ≈ 1000 kg/m^3)