Input interpretation

actinides | first molar ionization energy
Summary
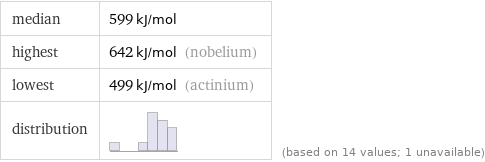
median | 599 kJ/mol highest | 642 kJ/mol (nobelium) lowest | 499 kJ/mol (actinium) distribution | | (based on 14 values; 1 unavailable)
Entity with missing value
lawrencium
Distribution plots
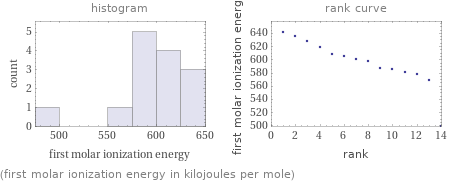
(first molar ionization energy in kilojoules per mole)
First molar ionization energy rankings
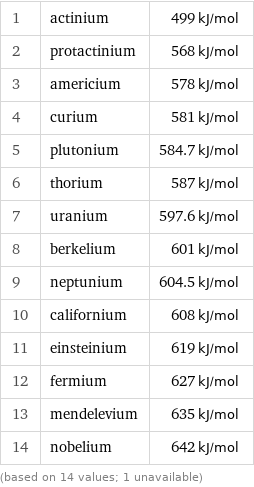
1 | actinium | 499 kJ/mol 2 | protactinium | 568 kJ/mol 3 | americium | 578 kJ/mol 4 | curium | 581 kJ/mol 5 | plutonium | 584.7 kJ/mol 6 | thorium | 587 kJ/mol 7 | uranium | 597.6 kJ/mol 8 | berkelium | 601 kJ/mol 9 | neptunium | 604.5 kJ/mol 10 | californium | 608 kJ/mol 11 | einsteinium | 619 kJ/mol 12 | fermium | 627 kJ/mol 13 | mendelevium | 635 kJ/mol 14 | nobelium | 642 kJ/mol (based on 14 values; 1 unavailable)
Unit conversions for median first molar ionization energy 599 kJ/mol
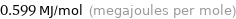
0.599 MJ/mol (megajoules per mole)
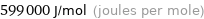
599000 J/mol (joules per mole)
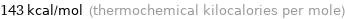
143 kcal/mol (thermochemical kilocalories per mole)
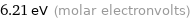
6.21 eV (molar electronvolts)
Comparisons for median first molar ionization energy 599 kJ/mol

≈ 0.25 × molar energy required to remove the first electron from helium ( 2372 kJ/mol )
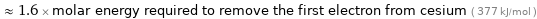
≈ 1.6 × molar energy required to remove the first electron from cesium ( 377 kJ/mol )