Input interpretation
selenious acid, dipotassium salt | nickel(II) cyanide tetrahydrate
Chemical names and formulas

| selenious acid, dipotassium salt | nickel(II) cyanide tetrahydrate formula | K_2SeO_3 | Ni(CN)_2·4H_2O Hill formula | K_2O_3Se | C_2H_8N_2NiO_4 name | selenious acid, dipotassium salt | nickel(II) cyanide tetrahydrate alternate names | dipotassium selenite | (none) mass fractions | K (potassium) 38.1% | O (oxygen) 23.4% | Se (selenium) 38.5% | C (carbon) 13.1% | H (hydrogen) 4.41% | N (nitrogen) 15.3% | Ni (nickel) 32.1% | O (oxygen) 35%
Structure diagrams
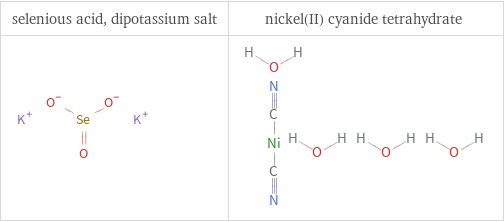
Structure diagrams
Basic properties
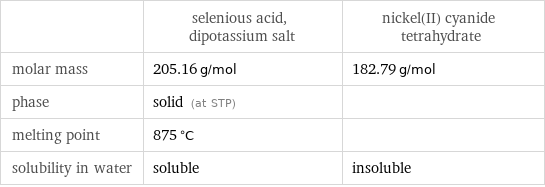
| selenious acid, dipotassium salt | nickel(II) cyanide tetrahydrate molar mass | 205.16 g/mol | 182.79 g/mol phase | solid (at STP) | melting point | 875 °C | solubility in water | soluble | insoluble
Units
