Input interpretation
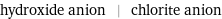
hydroxide anion | chlorite anion
Structure diagrams
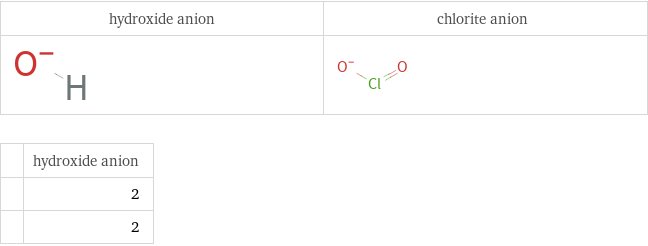
| hydroxide anion | 2 | 2
General properties
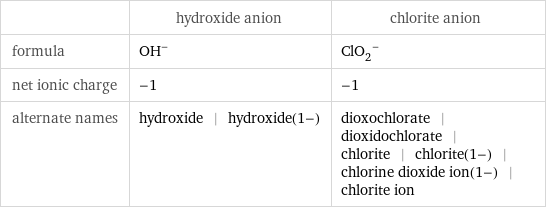
| hydroxide anion | chlorite anion formula | (OH)^- | (ClO_2)^- net ionic charge | -1 | -1 alternate names | hydroxide | hydroxide(1-) | dioxochlorate | dioxidochlorate | chlorite | chlorite(1-) | chlorine dioxide ion(1-) | chlorite ion
Ionic radii
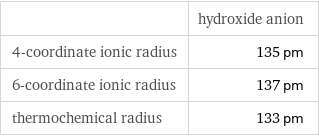
| hydroxide anion 4-coordinate ionic radius | 135 pm 6-coordinate ionic radius | 137 pm thermochemical radius | 133 pm
Units

Other properties
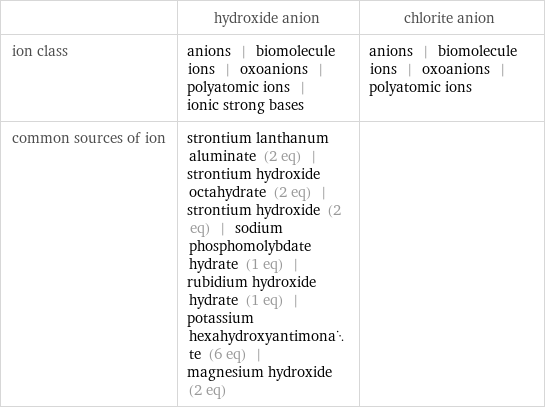
| hydroxide anion | chlorite anion ion class | anions | biomolecule ions | oxoanions | polyatomic ions | ionic strong bases | anions | biomolecule ions | oxoanions | polyatomic ions common sources of ion | strontium lanthanum aluminate (2 eq) | strontium hydroxide octahydrate (2 eq) | strontium hydroxide (2 eq) | sodium phosphomolybdate hydrate (1 eq) | rubidium hydroxide hydrate (1 eq) | potassium hexahydroxyantimonate (6 eq) | magnesium hydroxide (2 eq) |