Input interpretation

d-block elements | specific heat at STP
Summary
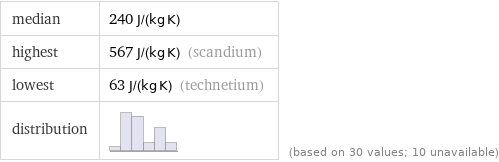
median | 240 J/(kg K) highest | 567 J/(kg K) (scandium) lowest | 63 J/(kg K) (technetium) distribution | | (based on 30 values; 10 unavailable)
Entities with missing values
lawrencium | rutherfordium | dubnium | seaborgium | bohrium | hassium | meitnerium | darmstadtium | roentgenium | copernicium (total: 10)
Distribution plots
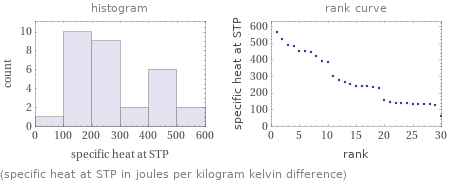
(specific heat at STP in joules per kilogram kelvin difference)
Specific heat at STP rankings
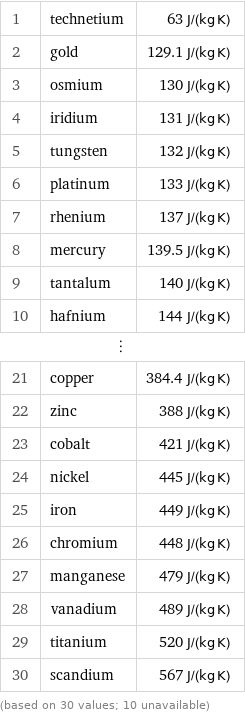
1 | technetium | 63 J/(kg K) 2 | gold | 129.1 J/(kg K) 3 | osmium | 130 J/(kg K) 4 | iridium | 131 J/(kg K) 5 | tungsten | 132 J/(kg K) 6 | platinum | 133 J/(kg K) 7 | rhenium | 137 J/(kg K) 8 | mercury | 139.5 J/(kg K) 9 | tantalum | 140 J/(kg K) 10 | hafnium | 144 J/(kg K) ⋮ | | 21 | copper | 384.4 J/(kg K) 22 | zinc | 388 J/(kg K) 23 | cobalt | 421 J/(kg K) 24 | nickel | 445 J/(kg K) 25 | iron | 449 J/(kg K) 26 | chromium | 448 J/(kg K) 27 | manganese | 479 J/(kg K) 28 | vanadium | 489 J/(kg K) 29 | titanium | 520 J/(kg K) 30 | scandium | 567 J/(kg K) (based on 30 values; 10 unavailable)
Unit conversions for median specific heat at STP 240 J/(kg K)
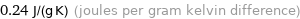
0.24 J/(g K) (joules per gram kelvin difference)
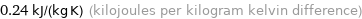
0.24 kJ/(kg K) (kilojoules per kilogram kelvin difference)
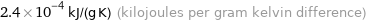
2.4×10^-4 kJ/(g K) (kilojoules per gram kelvin difference)

240 J/(kg °C) (joules per kilogram degree Celsius difference)
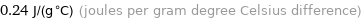
0.24 J/(g °C) (joules per gram degree Celsius difference)

0.0573 BTU_IT/(lb °F) (IT British thermal units per pound degree Fahrenheit difference)
Comparisons for median specific heat at STP 240 J/(kg K)
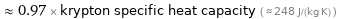
≈ 0.97 × krypton specific heat capacity ( ≈ 248 J/(kg K) )
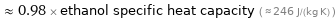
≈ 0.98 × ethanol specific heat capacity ( ≈ 246 J/(kg K) )
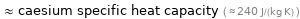
≈ caesium specific heat capacity ( ≈ 240 J/(kg K) )
Thermodynamic properties
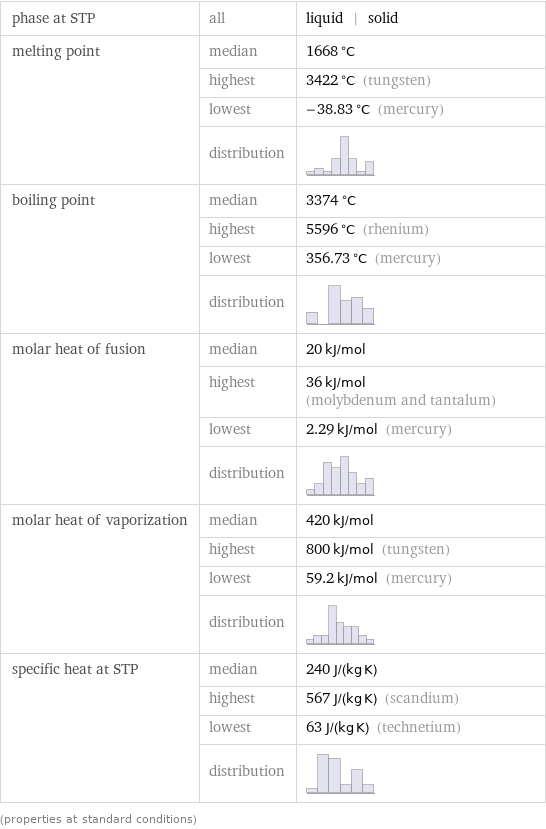
phase at STP | all | liquid | solid melting point | median | 1668 °C | highest | 3422 °C (tungsten) | lowest | -38.83 °C (mercury) | distribution | boiling point | median | 3374 °C | highest | 5596 °C (rhenium) | lowest | 356.73 °C (mercury) | distribution | molar heat of fusion | median | 20 kJ/mol | highest | 36 kJ/mol (molybdenum and tantalum) | lowest | 2.29 kJ/mol (mercury) | distribution | molar heat of vaporization | median | 420 kJ/mol | highest | 800 kJ/mol (tungsten) | lowest | 59.2 kJ/mol (mercury) | distribution | specific heat at STP | median | 240 J/(kg K) | highest | 567 J/(kg K) (scandium) | lowest | 63 J/(kg K) (technetium) | distribution | (properties at standard conditions)