Input interpretation
chromium(II) formate monohydrate | copper iodate
Chemical names and formulas
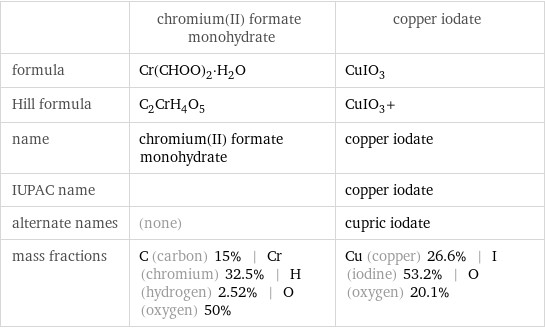
| chromium(II) formate monohydrate | copper iodate formula | Cr(CHOO)_2·H_2O | CuIO_3 Hill formula | C_2CrH_4O_5 | CuIO_3+ name | chromium(II) formate monohydrate | copper iodate IUPAC name | | copper iodate alternate names | (none) | cupric iodate mass fractions | C (carbon) 15% | Cr (chromium) 32.5% | H (hydrogen) 2.52% | O (oxygen) 50% | Cu (copper) 26.6% | I (iodine) 53.2% | O (oxygen) 20.1%
Structure diagrams
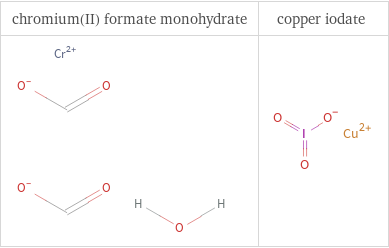
Structure diagrams
Basic properties
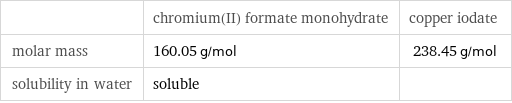
| chromium(II) formate monohydrate | copper iodate molar mass | 160.05 g/mol | 238.45 g/mol solubility in water | soluble |
Units
