Input interpretation

phosphine | tribromosilane
Chemical names and formulas

| phosphine | tribromosilane formula | PH_3 | SiHBr_3 Hill formula | H_3P | Br_3HSi name | phosphine | tribromosilane IUPAC name | phosphine | alternate names | celphos | detia gas ex-b | hydrogen phosphide | phosphane | phosphene | (none) mass fractions | H (hydrogen) 8.89% | P (phosphorus) 91.1% | Br (bromine) 89.2% | H (hydrogen) 0.375% | Si (silicon) 10.4%
Structure diagrams

| phosphine | tribromosilane vertex count | 1 | 4 edge count | 3 | 4 Schultz index | 0 | 36 Wiener index | 0 | 9 Hosoya index | 1 | 4 Balaban index | 0 | 2.324
3D structure
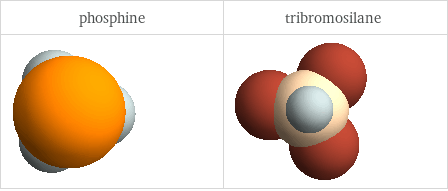
3D structure
Basic properties

| phosphine | tribromosilane molar mass | 33.998 g/mol | 268.8 g/mol phase | gas (at STP) | melting point | -132.8 °C | -73 °C boiling point | -87.5 °C | 109 °C density | 0.00139 g/cm^3 | 2.7 g/cm^3 solubility in water | slightly soluble | reacts
Units

Gas properties
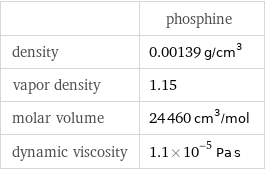
| phosphine density | 0.00139 g/cm^3 vapor density | 1.15 molar volume | 24460 cm^3/mol dynamic viscosity | 1.1×10^-5 Pa s
Units

Thermodynamic properties
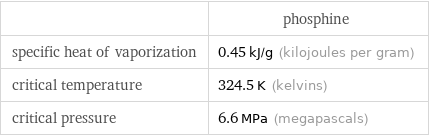
| phosphine specific heat of vaporization | 0.45 kJ/g (kilojoules per gram) critical temperature | 324.5 K (kelvins) critical pressure | 6.6 MPa (megapascals)