Input interpretation

phosphide anion | ion class
Result
anions | group 15 ions | ionic ligands | monatomic anions | p block ions
Ionic radii
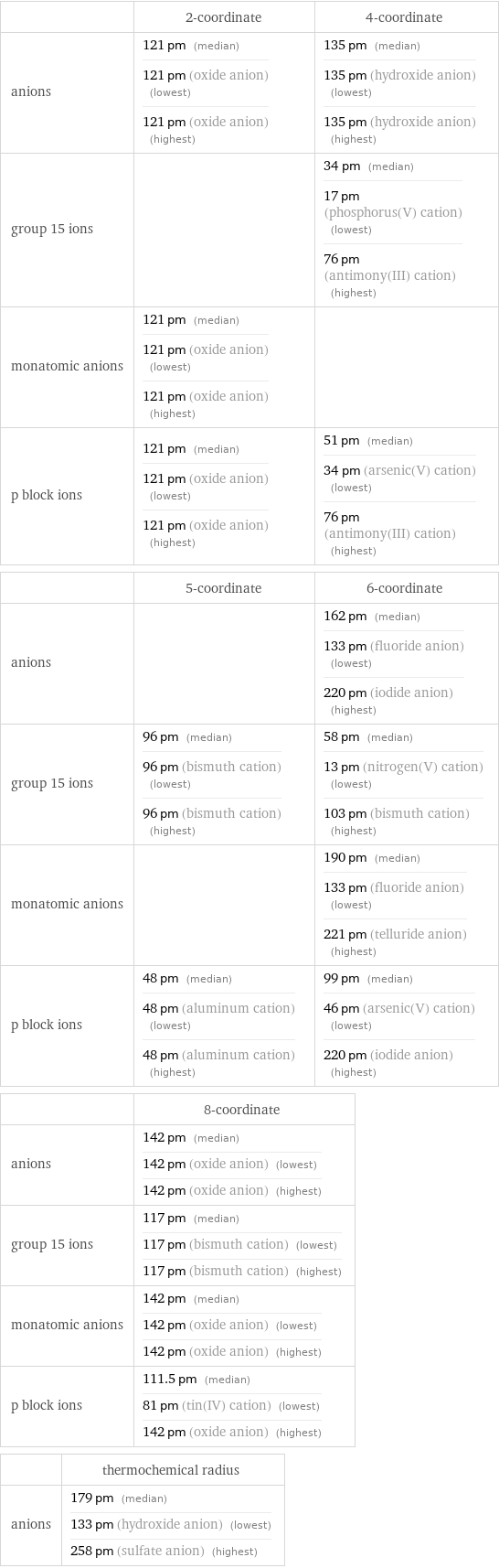
| 2-coordinate | 4-coordinate anions | 121 pm (median) 121 pm (oxide anion) (lowest) 121 pm (oxide anion) (highest) | 135 pm (median) 135 pm (hydroxide anion) (lowest) 135 pm (hydroxide anion) (highest) group 15 ions | | 34 pm (median) 17 pm (phosphorus(V) cation) (lowest) 76 pm (antimony(III) cation) (highest) monatomic anions | 121 pm (median) 121 pm (oxide anion) (lowest) 121 pm (oxide anion) (highest) | p block ions | 121 pm (median) 121 pm (oxide anion) (lowest) 121 pm (oxide anion) (highest) | 51 pm (median) 34 pm (arsenic(V) cation) (lowest) 76 pm (antimony(III) cation) (highest) | 5-coordinate | 6-coordinate anions | | 162 pm (median) 133 pm (fluoride anion) (lowest) 220 pm (iodide anion) (highest) group 15 ions | 96 pm (median) 96 pm (bismuth cation) (lowest) 96 pm (bismuth cation) (highest) | 58 pm (median) 13 pm (nitrogen(V) cation) (lowest) 103 pm (bismuth cation) (highest) monatomic anions | | 190 pm (median) 133 pm (fluoride anion) (lowest) 221 pm (telluride anion) (highest) p block ions | 48 pm (median) 48 pm (aluminum cation) (lowest) 48 pm (aluminum cation) (highest) | 99 pm (median) 46 pm (arsenic(V) cation) (lowest) 220 pm (iodide anion) (highest) | 8-coordinate anions | 142 pm (median) 142 pm (oxide anion) (lowest) 142 pm (oxide anion) (highest) group 15 ions | 117 pm (median) 117 pm (bismuth cation) (lowest) 117 pm (bismuth cation) (highest) monatomic anions | 142 pm (median) 142 pm (oxide anion) (lowest) 142 pm (oxide anion) (highest) p block ions | 111.5 pm (median) 81 pm (tin(IV) cation) (lowest) 142 pm (oxide anion) (highest) | thermochemical radius anions | 179 pm (median) 133 pm (hydroxide anion) (lowest) 258 pm (sulfate anion) (highest)