Input interpretation
ammonium nitrite | m-carborane
Chemical names and formulas
![| ammonium nitrite | m-carborane formula | H_4N_2O_2 | C_2H_12B_10 Hill formula | H_4N_2O_2 | B_10C_2H_12 name | ammonium nitrite | m-carborane IUPAC name | azanium nitrite | alternate names | ammonium nitrite [Forbidden] | nitrous acid, ammonium salt | 1, 7-dicarbadodecaborane(12) | 2\!\(\*SuperscriptBox[\(λ\), \(2\)]\), 3\!\(\*SuperscriptBox[\(λ\), \(2\)]\), 4\!\(\*SuperscriptBox[\(λ\), \(2\)]\), 5\!\(\*SuperscriptBox[\(λ\), \(2\)]\), 6\!\(\*SuperscriptBox[\(λ\), \(2\)]\), 7\!\(\*SuperscriptBox[\(λ\), \(2\)]\), 8\!\(\*SuperscriptBox[\(λ\), \(2\)]\), 9\!\(\*SuperscriptBox[\(λ\), \(2\)]\), 11\!\(\*SuperscriptBox[\(λ\), \(2\)]\), 12\!\(\*SuperscriptBox[\(λ\), \(2\)]\)-decaborabicyclo[8.1.1]dodecane mass fractions | H (hydrogen) 6.3% | N (nitrogen) 43.7% | O (oxygen) 50% | B (boron) 80.6% | C (carbon) 17.9% | H (hydrogen) 1.5%](../image_source/2bec8a2416929b307eadd85827f93fb6.png)
| ammonium nitrite | m-carborane formula | H_4N_2O_2 | C_2H_12B_10 Hill formula | H_4N_2O_2 | B_10C_2H_12 name | ammonium nitrite | m-carborane IUPAC name | azanium nitrite | alternate names | ammonium nitrite [Forbidden] | nitrous acid, ammonium salt | 1, 7-dicarbadodecaborane(12) | 2\!\(\*SuperscriptBox[\(λ\), \(2\)]\), 3\!\(\*SuperscriptBox[\(λ\), \(2\)]\), 4\!\(\*SuperscriptBox[\(λ\), \(2\)]\), 5\!\(\*SuperscriptBox[\(λ\), \(2\)]\), 6\!\(\*SuperscriptBox[\(λ\), \(2\)]\), 7\!\(\*SuperscriptBox[\(λ\), \(2\)]\), 8\!\(\*SuperscriptBox[\(λ\), \(2\)]\), 9\!\(\*SuperscriptBox[\(λ\), \(2\)]\), 11\!\(\*SuperscriptBox[\(λ\), \(2\)]\), 12\!\(\*SuperscriptBox[\(λ\), \(2\)]\)-decaborabicyclo[8.1.1]dodecane mass fractions | H (hydrogen) 6.3% | N (nitrogen) 43.7% | O (oxygen) 50% | B (boron) 80.6% | C (carbon) 17.9% | H (hydrogen) 1.5%
Structure diagrams
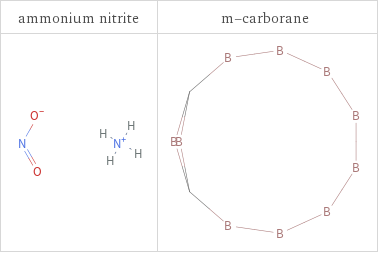
Structure diagrams
Basic properties

| ammonium nitrite | m-carborane molar mass | 64.04 g/mol | 134.1 g/mol solubility in water | soluble |
Units
